CYP3A4 genotype is associated with sildenafil concentrations in patients with heart failure with preserved ejection fraction
- PMID: 28440343
- PMCID: PMC5656562
- DOI: 10.1038/tpj.2017.8
CYP3A4 genotype is associated with sildenafil concentrations in patients with heart failure with preserved ejection fraction
Abstract
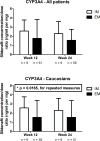
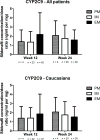
Despite its established inter-individual variability, sildenafil has been the subject of only a few pharmacogenetic investigations, with limited data regarding the genetic modulators of its pharmacokinetics. We conducted a pharmacogenetic sub-study of patients randomized to sildenafil (n=85) in the RELAX trial, which investigated the impact of high-dose sildenafil in patients with heart failure with preserved left ventricular ejection fraction (HFpEF). In the overall population, the CYP3A4 inferred phenotype appeared associated with the dose-adjusted peak concentrations of sildenafil at week 12 and week 24 (adjusted P=0.045 for repeated measures analysis), although this P-value did not meet our corrected significance threshold of 0.0167. In the more homogeneous Caucasian subgroup, this association was significant (adjusted P=0.0165 for repeated measures). Hence, CYP3A4 inferred phenotype is associated with peak sildenafil dose-adjusted concentrations in patients with HFpEF receiving high doses of sildenafil. The clinical impact of this association requires further investigation.
Conflict of interest statement
Simon de Denus has received research grants or been a coinvestigator on grants supported by AstraZeneca, Novartis, Roche and Pfizer. He has received speaker fees from Pfizer and consulting fees from Servier and Novartis. Jean Lucien Rouleau is a consultant for Novartis. Marie-Pierre Dubé has been a coinvestigator on grants supported by AstraZeneca and Pfizer, has received honoraria and research contracts from Roche and is a member of the executive committee of the Dal-GenE trial sponsored by DalCor. A patent was submitted on pharmacogenomic determinants of responses to cardiovascular drugs and Dr Dubé is listed as inventor.
Figures

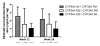
References
-
- Kanjanawart S, Gaysonsiri D, Tangsucharit P, Vannaprasaht S, Phunikhom K, Kaewkamson T, et al. Comparative bioavailability of two sildenafil tablet formulations after single-dose administration in healthy Thai male volunteers. Int J Clin Pharmacol Ther. 2011;49:525–530. - PubMed
-
- Shon JH, Ku HY, Bae SY, Oh MK, Yeo CW, Bae SK, et al. The disposition of three phosphodiesterase type 5 inhibitors, vardenafil, sildenafil, and udenafil, is differently influenced by the CYP3A5 genotype. Pharmacogenet Genomics. 2011;21:820–828. - PubMed
-
- Benard F, Carrier S, Lee JC, Talwar V, Defoy I. Men with mild erectile dysfunction benefit from sildenafil treatment. J Sex Med. 2010;7:3725–3735. - PubMed
-
- Muniz JJ, Lacchini R, Rinaldi TO, Nobre YT, Cologna AJ, Martins AC, et al. Endothelial nitric oxide synthase genotypes and haplotypes modify the responses to sildenafil in patients with erectile dysfunction. Pharmacogenomics J. 2013;13:189–196. - PubMed
-
- Eisenhardt A, Sperling H, Hauck E, Porst H, Stief C, Rubben H, et al. ACE gene I/D and NOS3 G894T polymorphisms and response to sildenafil in men with erectile dysfunction. Urology. 2003;62:152–157. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

