Improved gene delivery to adult mouse spinal cord through the use of engineered hybrid adeno-associated viral serotypes
- PMID: 28440798
- PMCID: PMC5472488
- DOI: 10.1038/gt.2017.27
Improved gene delivery to adult mouse spinal cord through the use of engineered hybrid adeno-associated viral serotypes
Abstract
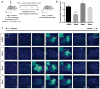
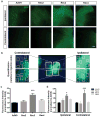
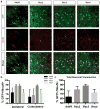
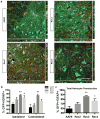
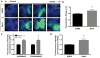
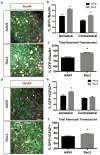
Adeno-associated viral (AAV) vectors are often used in gene therapy for neurological disorders because of its safety profile and promising results in clinical trials. One challenge to AAV gene therapy is effective transduction of large numbers of the appropriate cell type, which can be overcome by modulating the viral capsid through DNA shuffling. Our previous study demonstrates that Rec2, among a family of novel engineered hybrid capsid serotypes (Rec1~4) transduces adipose tissue with far superior efficiency than naturally occurring AAV serotypes. Here we assessed the transduction of adult spinal cord at two different doses of AAV vectors expressing green fluorescent protein (2 × 109 or 4 × 108 viral particles) via intraparenchymal injection at the thoracic vertebral level T9. In comparison with an equal dose of the currently preferable AAV9 serotype, Rec3 serotype transduced a broader region of the spinal cord up to ~1.5 cm longitudinally and displayed higher transgene expression and increased maximal transduction rates of astrocytes at either dose and neurons at the lower dose. These novel engineered hybrid vectors could provide powerful tools at lower production costs to manipulate gene expression in the spinal cord for mechanistic studies or provide potent vehicles for gene therapy delivery, such as neurotrophins, to the spinal cord.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Mingozzi F, High KA. Therapeutic in vivo gene transfer for genetic disease using AAV: progress and challenges. Nature reviews Genetics. 2011;12(5):341–55. - PubMed
-
- Mizukami H, Mimuro J, Ogura T, Okada T, Urabe M, Kume A, et al. Adipose tissue as a novel target for in vivo gene transfer by adeno-associated viral vectors. Human gene therapy. 2006;17(9):921–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

