Antibiotic strategies for eradicating Pseudomonas aeruginosa in people with cystic fibrosis
- PMID: 28440853
- PMCID: PMC6478104
- DOI: 10.1002/14651858.CD004197.pub5
Antibiotic strategies for eradicating Pseudomonas aeruginosa in people with cystic fibrosis
Update in
-
Antibiotic strategies for eradicating Pseudomonas aeruginosa in people with cystic fibrosis.Cochrane Database Syst Rev. 2023 Jun 2;6(6):CD004197. doi: 10.1002/14651858.CD004197.pub6. Cochrane Database Syst Rev. 2023. PMID: 37268599 Free PMC article. Review.
Abstract
Background: Respiratory tract infection with Pseudomonas aeruginosa occurs in most people with cystic fibrosis. Once chronic infection is established, Pseudomonas aeruginosa is virtually impossible to eradicate and is associated with increased mortality and morbidity. Early infection may be easier to eradicate.This is an update of a Cochrane review first published in 2003, and previously updated in 2006, 2009 and 2014.
Objectives: To determine whether antibiotic treatment of early Pseudomonas aeruginosa infection in children and adults with cystic fibrosis eradicates the organism, delays the onset of chronic infection, and results in clinical improvement. To evaluate whether there is evidence that a particular antibiotic strategy is superior to or more cost-effective than other strategies and to compare the adverse effects of different antibiotic strategies (including respiratory infection with other micro-organisms).
Search methods: We searched the Cochrane Cystic Fibrosis and Genetic Disorders Group Trials Register comprising references identified from comprehensive electronic database searches and handsearches of relevant journals and abstract books of conference proceedings.Most recent search: 10 October 2016.
Selection criteria: We included randomised controlled trials of people with cystic fibrosis, in whom Pseudomonas aeruginosa had recently been isolated from respiratory secretions. We compared combinations of inhaled, oral or intravenous antibiotics with placebo, usual treatment or other combinations of inhaled, oral or intravenous antibiotics. We excluded non-randomised trials, cross-over trials, and those utilising historical controls.
Data collection and analysis: Both authors independently selected trials, assessed risk of bias and extracted data.
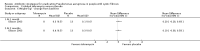
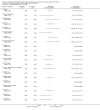
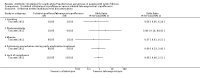
Main results: The search identified 60 trials; seven trials (744 participants) with a duration between 28 days and 27 months were eligible for inclusion. Three of the trials are over 10 years old and their results may be less applicable today given the changes in standard treatment. Some of the trials had low numbers of participants and most had relatively short follow-up periods; however, there was generally a low risk of bias from missing data. In most trials it was difficult to blind participants and clinicians to treatment given the interventions and comparators used. Two trials were supported by the manufacturers of the antibiotic used.Evidence from two trials (38 participants) at the two-month time-point showed treatment of early Pseudomonas aeruginosa infection with inhaled tobramycin results in microbiological eradication of the organism from respiratory secretions more often than placebo, odds ratio 0.15 (95% confidence interval (CI) 0.03 to 0.65) and data from one of these trials, with longer follow up, suggested that this effect may persist for up to 12 months.One randomised controlled trial (26 participants) compared oral ciprofloxacin and nebulised colistin versus usual treatment. Results after two years suggested treatment of early infection results in microbiological eradication of Pseudomonas aeruginosa more often than no anti-pseudomonal treatment, odds ratio 0.12 (95% CI 0.02 to 0.79).One trial comparing 28 days to 56 days treatment with nebulised tobramycin solution for inhalation in 88 participants showed that both treatments were effective and well-tolerated, with no notable additional improvement with longer over shorter duration of therapy. However, this trial was not powered to detect non-inferiority or equivalence .A trial of oral ciprofloxacin with inhaled colistin versus nebulised tobramycin solution for inhalation alone (223 participants) failed to show a difference between the two strategies, although it was underpowered to show this. A further trial of inhaled colistin with oral ciprofloxacin versus nebulised tobramycin solution for inhalation with oral ciprofloxacin also showed no superiority of the former, with increased isolation of Stenotrophomonas maltophilia in both groups.A recent, large trial in 306 children aged between one and 12 years compared cycled nebulised tobramycin solution for inhalation to culture-based therapy and also ciprofloxacin to placebo. The primary analysis showed no difference in time to pulmonary exacerbation or proportion of Pseudomonas aeruginosa positive cultures. An analysis performed in this review (not adjusted for age) showed fewer participants in the cycled therapy group with one or more isolates of Pseudomonas aeruginosa, odds ratio 0.51 (95% CI 0.31 to 0.28). Using GRADE, the quality of evidence for outcomes was downgraded to moderate to very low. Downgrading decisions for Pseudomonas aeruginosa eradication and lung function were based on applicability (participants mostly children) and limitations in study design, with imprecision an additional limitation for lung function, growth parameters and adverse effects.
Authors' conclusions: We found that nebulised antibiotics, alone or in combination with oral antibiotics, were better than no treatment for early infection with Pseudomonas aeruginosa. Eradication may be sustained for up to two years. There is insufficient evidence to determine whether antibiotic strategies for the eradication of early Pseudomonas aeruginosa decrease mortality or morbidity, improve quality of life, or are associated with adverse effects compared to placebo or standard treatment. Four trials comparing two active treatments have failed to show differences in rates of eradication of Pseudomonas aeruginosa. There have been no published randomised controlled trials that investigate the efficacy of intravenous antibiotics to eradicate Pseudomonas aeruginosa in cystic fibrosis. Overall, there is still insufficient evidence from this review to state which antibiotic strategy should be used for the eradication of early Pseudomonas aeruginosa infection in cystic fibrosis.
Conflict of interest statement
Dr Langton Hewer is the lead investigator on the ongoing trial Torpedo‐CF: Trial of Optimal Therapy for Pseudomonas Eradication in Cystic Fibrosis.
Prof Smyth declares relevant activities of membership of a Raptor steering committee, consultancies for Raptor, Gilead, Vertex, Roche and PTC. Actavis provide support for CF team educational activities.
Figures





















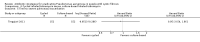














Update of
-
Antibiotic strategies for eradicating Pseudomonas aeruginosa in people with cystic fibrosis.Cochrane Database Syst Rev. 2014 Nov 10;(11):CD004197. doi: 10.1002/14651858.CD004197.pub4. Cochrane Database Syst Rev. 2014. Update in: Cochrane Database Syst Rev. 2017 Apr 25;4:CD004197. doi: 10.1002/14651858.CD004197.pub5. PMID: 25383937 Updated.
References
References to studies included in this review
Gibson 2003 {published data only}
-
- Gibson RL, Emerson J, McNamara S, Burns JL, Rosenfeld M, Yunker A, et al. A randomized, controlled trial of inhaled tobramycin in young children with cystic fibrosis: Eradication of pseudomonas from the lower airway. Pediatric Pulmonology 2002;Suppl 24:300. [CFGD Register: PI151c]
-
- Gibson RL, Emerson J, McNamara S, Burns JL, Rosenfeld M, Yunker A, et al. Online Supplement to 'Significant microbiological effect of inhaled tobramycin in young children with cystic fibrosis'. [online]. American Journal of Respiratory and Critical Care Medicine 2003;167(6):841 Online. [CFGD Register: PI151e] - PubMed
-
- Gibson RL, Emerson J, McNamara S, Burns JL, Rosenfeld M, Yunker A, et al. Significant microbiological effect of inhaled tobramycin in young children with cystic fibrosis. American Journal of Respiratory and Critical Care Medicine 2003;167(6):841‐9. [CFGD Register: PI151d] - PubMed
-
- Rosenfeld M. Serum and lower respiratory tract tobramycin concentrations produced by inhaled tobramycin (TOBI) in young children with cystic fibrosis. Pediatric Pulmonology 1999;Suppl 19:106‐8. [CFGD Register: PI151b]
-
- Rosenfeld M, Borowitz D, Emerson J, Gibson R, McCoy K, McNamara S, et al. Serum pharmacokinetics and safety of inhaled tobramycin in very young CF patients. Pediatric Pulmonology 1999;Suppl 19:262. [CFGD Register: PI151a]
Proesmans 2013 {published data only}
-
- Proesmans M, Boulanger L, Vermeulen F, Boeck K. Eradication of recent Pseudomonas aeruginosa isolation: TOBI versus colistin/ciprofloxacin. Journal of Cystic Fibrosis 2008;7(S2):S64. [CFGD Register: PI208a]
-
- Proesmans M, Boulanger L, Vermeulen F, Boeck K. Eradication of recent Pseudomonas aeruginosa isolation: TOBI versus colistin/ciprofloxacin. Pediatric Pulmonology 2009;44(S32):321. [Abstract no: 311; CFGD Register: PI208b; MEDLINE: ]
-
- Proesmans M, Boulanger L, Vermeulen F, Boeck K. Eradication of recent pseudomonas aeruginosa infection: TOBI versus Colistineb®/ ciprofloxacin. Journal of Cystic Fibrosis 2011;10 Suppl 1:S26. [Abstract no: 102; CFGD Register: PI208c]
-
- Proesmans M, Vermeulen F, Boulanger L, Verhaegen J, Boeck K. Comparison of two treatment regimens for eradication of Pseudomonas aeruginosa infection in children with cystic fibrosis. Journal of Cystic Fibrosis 2013;12(1):29‐34. [CFGD Register: PI208d] - PubMed
Ratjen 2010 {published data only}
-
- Ratjen F, Munck A, Campello V. Inhaled tobramycin nebuliser solution for treatment of early Pseudomonas aeruginosa infection: first results from the Elite study. Pediatric Pulmonlogy 2006;41(S29):318. [CFGD Register: PI197b]
-
- Ratjen F, Munck A, Campello V. Safety of inhaled tobramycin nebuliser solution for treatment of early pseudomonas aeruginosa infection: first results from the ELITE study. Journal of Cystic Fibrosis 2006;5 Suppl 1:S22. [CFGD Register: PI197a]
-
- Ratjen F, Munck A, Kho P. Short and long‐term efficacy of inhaled tobramycin in early P. Aeruginosa infection: the ELITE study. Pediatric Pulmonology 2008;43(S31):319. [CFGD Register: PI197d]
-
- Ratjen F, Munck A, Kho P, Angyalosi G, for the ELITE Study Group. Treatment of early Pseudomonas aeruginosa infection in patients with cystic fibrosis: the ELITE trial. Thorax 2010;65(4):286‐91. [CFGD Register: PI197e] - PubMed
-
- Ratjen F, Stenglein S, Munck A. Inhaled tobramycin nebulizer solution for treatment of early Pseudomonas aeruginosa infection; the ELITE study. Journal of Cystic Fibrosis 2008;7 Suppl 2:S26. [CFGD Register: PI197c]
Taccetti 2012 {published data only}
-
- Cariani L, Defilippi G, Costantini D, Claut L, Clarizia G, D'accico M, et al. Semi‐automated rep‐pcr genotyping of pseudomonas aeruginosa in Italian CF patients in eradication therapy. Pediatric Pulmonology 2010;45 Suppl 33:348. [Abstract no: 360; CFGD Register: PI230c]
-
- Dolce D, Cariani L, Ravenni N, Mergni G, Biffi A, Colombo C, et al. Anti‐P.aeruginosa antibodies and microbiological outcome in patients treated with early eradication therapy. Pediatric Pulmonology 2013;48 Suppl 36:288. [Abstract no: 231; CENTRAL: 921692; CFGD Register: PI230i; CRS: 5500125000000403]
-
- Taccetti G, Bianchini E, Zavataro L, Campana S, Defilippi G, Ravenni N, et al. Pseudomonas aeruginosa eradication in cystic fibrosis: preliminary data from a randomized multicenter study of two different early antibiotic treatment protocols. Pediatric Pulmonology 2010;45(S33):337. [Abstract no: 332; CFGD Register: PI230d]
-
- Taccetti G, Bianchini E, Zavataro L, Campana S, Defilippi G, Ravenni N, et al. Pseudomonas aeruginosa microbiological status and emergence of other pathogens after early eradication treatment in cystic fibrosis: a post‐trial follow‐up. Pediatric Pulmonology 2011;46(S34):317. [Abstract no: 292; CFGD Register: PI230e]
-
- Taccetti G, Bianchini E, Zavataro L, Campana S, Ravenni N, Boni V, et al. Early antibiotic treatment for Pseudomonas aeruginosa eradication in cystic fibrosis patients: a randomized multicenter study of two different protocols. Pediatric Pulmonology 2009;44(S32):354. [Abstract no: 406; CFGD CF Register: PI230a; MEDLINE: ]
Treggiari 2011 {published data only}
-
- Anstead M, Lymp J, Khan U, Barbieri J, Langkamp M, Doring G, et al. Pseudomonas aeruginosa serology predicts response to treatment and re‐infection in the EPIC clinical study. Pediatric Pulmonology 2011;46(S34):303. [Abstract no: 254; CFGD Register: PI202g]
-
- Hamblett NM, Retsch‐Bogart GZ, Treggiari M, Kronmal RA, Khan U, Williams J, et al. Safety and efficacy of anti‐pseudomonal therapy for early eradication of Pseudomonas aeruginosa: the EPIC study. Pediatric Pulmonology 2009;44(S32):183. [CFGD CF Register: PI202b; MEDLINE: ]
-
- Hoffman LR, Ramsey BW, Kulasekara HD, Retsch‐Bogart GZ, Wolter DJ, Pope CE, et al. Pseudomonas aeruginosa (PA) phenotypes associated with persistent early infection in CF patients in the EPIC Clinical Trial. Pediatric Pulmonology 2012;47(S35):317. [Abstract no: 266; CFGD Register: PI202j]
-
- Jorth P, Hisert KB, Garudathri J, Wolter D, Hoffman L, Singh P. Studies on the effects of ciprofloxacin on pseudomonas aeruginosa evolution in cystic fibrosis patients. Pediatric Pulmonology 2014;49:349. [CENTRAL: 1057036; CFGD Register: PI202n; CRS: 5500050000000261; EMBASE: 71616423]
Valerius 1991 {published data only}
-
- Valerius NH, Koch C, Høiby N. Prevention of chronic Pseudomonas aeruginosa colonisation in cystic fibrosis by early treatment. Lancet 1991;338(8769):725‐6. [CFGD Register: PI70b] - PubMed
-
- Valerius NH, Koch C, Høiby N. Prevention of chronic colonization with Pseudomonas aeruginosa in patients with CF by early treatment with ciprofloxacin and colistin aerosol inhalations. Pediatric Pulmonology 1990;9(Supplement S5):248. [CFGD Register: PI70a]
Wiesemann 1998 {published data only}
-
- Ratjen F, Steinkamp G, Döring G, Bauernfeind A, Wiesemann HG, Hardt H. Prevention of chronic pseudomonas aeruginosa infection by early inhalation therapy with tobramycin. Pediatric Pulmonology 1994;Suppl 10:250. [CFGD Register: PI101a]
-
- Wiesemann HG, Steinkamp G, Ratjen F, Baurnfeind A, Przyklenk B, Döring G, et al. Placebo‐controlled, double‐blind, randomised study of aerosolised tobramycin for early treatment of Pseudomonas aeruginosa colonization in cystic fibrosis. Pediatric Pulmonology 1998;25(2):88‐92. [CFGD Register: PI101b] - PubMed
References to studies excluded from this review
Alothman 2002 {published data only}
-
- Alothman GA, Alsaadi MM, Ho BL, Ho SL, Dupuis A, Corey M, et al. Evaluation of bronchial constriction in children with cystic fibrosis after inhaling two different preparations of tobramycin. Chest 2002;122(3):930‐4. [CENTRAL: 398028; CFGD Register: PI157b; CRS: 5500100000002196; PUBMED: 12226034] - PubMed
-
- Alothman GA, Coates AL, Corey M, Dupuis A, Ho SL, Ho BL, et al. In cystic fibrosis (CF) patients, does the inhalation of an intravenous tobramycin preparation result in more bronchospasm than a preservative free tobramycin preparation?. Pediatric Pulmonology 2000;Suppl 20:298‐9. [CFGD Register: PI157a]
Alothman 2005 {published data only}
-
- Alothman GA, Ho B, Alsaadi MM, Ho SL, O'Drowsky L, Louca E, et al. Bronchial constriction and inhaled colistin in cystic fibrosis. Chest 2005;127(2):522‐9. [CENTRAL: 502709; CFGD Register: PI190; CRS: 5500100000002670; PUBMED: 15705991] - PubMed
Ballman 1998 {published data only}
Brett 1992 {published data only}
Church 1997 {published data only}
-
- Church DA, Kanga JF, Kuhn RJ, Rubio TT, Spohn WA, Stevens JC, et al. Sequential ciprofloxacin therapy in pediatric cystic fibrosis: comparative study vs. ceftazidime/tobramycin in the treatment of acute pulmonary exacerbations. The Cystic Fibrosis Study Group. Pediatric Infectious Disease Journal 1997;16(1):97‐105. [CENTRAL: 135891; CFGD Register: PI115; CRS: 5500100000000803; PUBMED: 9002118] - PubMed
Clancy 2013 {published data only}
-
- Clancy JP, Dupont L, Konstan MW, Billings J, Fustik S, Goss CH, et al. Online supplementary data to "Phase II studies of nebulised Arikace in CF patients with Pseudomonas aeruginosa infection" [online. Thorax 2013;68(9):818‐25 online. [CFGD Register: PI207f // PI222d ; CRS: 5500135000000003] - PMC - PubMed
-
- Clancy JP, Minic P, Dupont L, Goss CH, Quittner AL, Lymp JF, et al. Full analysis of data from two phase II blinded & placebo‐controlled studies of nebulized liposomal amikacin for inhalation (Arikace) in the treatment of CF patients with pseudomonas aeruginosa lung infection. Pediatric Pulmonology 2010;45 Suppl 33:299. [Abstract no: 227; CENTRAL: 848916; CFGD Register: PI207d // PI222b; CRS: 5500100000010628]
-
- Dupont L, Minic P, Fustic S, Mazurek H, Solyom E, Feketova A, et al. A randomised placebo‐controlled study of nebulized liposomal amikacin (Arikace) in the treatment of cystic fibrosis patients with chronic Pseudomonas aeruginosa lung infection. Journal of Cystic Fibrosis 2008;7 Suppl 2:S26. [CENTRAL: 790820; CFGD Register: PI207a; CRS: 5500100000003531]
-
- Dupont LJ, Minic P, Fustik S, Mazurek H, Solyom E, Feketeova A, et al. A randomized placebo‐controlled study of nebulized liposomal amikacin (Arikace) in the treatment of cystic fibrosis patients with chronic Pseudomonas Aeruginosa lung infection. Pediatric Pulmonology 2008;43 Suppl 31:301. [CENTRAL: 790939; CFGD Register: PI207b; CRS: 5500100000003535]
Coates 2011 {published data only}
-
- Coates AL, Denk O, Leung K, Ribeiro N, Chan J, Green M, et al. Higher tobramycin concentration and vibrating mesh technology can shorten antibiotic treatment time in cystic fibrosis. Pediatric Pulmonology 2011;46(4):401‐8. [CENTRAL: 786190; CFGD Register: PI241b; CRS: 5500100000006333] - PubMed
-
- Denk O, Caotes AL, Keller M, Leung K, Green M, Chan J, et al. Lung delivery of a new tobramycin nebuliser solution (150mg/1.5ml) by an investigational eFlow® nebuliser is equivalent to TOBI® but in a fraction of time. Journal of Cystic Fibrosis 2009;8 Suppl 2:S66. [Abstract no: 264; CENTRAL: 794467; CFGD Register: PI241c; CRS: 5500100000003576]
-
- Keller M, Coates AL, Griese M, Denk O, Schierholz J, Knoch M. In‐vivo data support equivalent therapeutic efficacy of a new tobramycin inhalation solution (150mg/1.5ml) administered by the eFlow® electronic nebuliser compared to TOBI® in the PARI LC PLUS®. Journal of Cystic Fibrosis 2010;9 Suppl 1:S22. [Abstract no: 84; CENTRAL: 794286; CFGD Register: PI241a; CRS: 5500100000003569]
Elborn 2015 {published data only}
-
- Elborn JS, Flume PA, Cohen F, Loutit J, VanDevanter DR. Safety and efficacy of prolonged levofloxacin inhalation solution (APT‐1026)treatment for cystic fibrosis and chronic Pseudomonas aeruginosa airway infection. Journal of Cystic Fibrosis 2016;15(5):634‐40. [CFGD Register: PI262d] - PubMed
-
- Elborn JS, Flume PA, Loutit J, Cohen F. Prolonged improvement in lung function and quality of life in cystic fibrosis: a 24‐week extension study of levofloxacin nebulization solution (APT‐1026) versus tobramycin nebulization solution in stable CF patients with chronic pseudomonas aeruginosa infection. Journal of Cystic Fibrosis 2014;13 Suppl 2:S16. [Abstract no: WS7.5; CENTRAL: 1000055; CFGD Register: PI262b; CRS: 5500131000000008]
-
- Elborn JS, Geller D, Conrad D, Aaron S, Smyth AR, Fischer R, et al. Phase 3 trial of inhaled levofloxacin (Aeruquinâ™, MP‐376, APT‐1026) vs. tobramycin inhalation solution (TIS) in intensively treated CF patients over 6 months. Journal of Cystic Fibrosis 2013;12 Suppl 1:S35. [Abstract no: WS17.6; CENTRAL: 867323; CFGD Register: PI262a; CRS: 5500100000011291]
-
- Elborn JS, Geller DE, Conrad D, Aaron SD, Smyth AR, Fischer R, et al. A phase 3, open‐label, randomized trial to evaluate the safety and efficacy of levofloxacin inhalation solution (APT‐1026) versus tobramycin inhalation solution in stable cystic fibrosis patients. Journal of Cystic Fibrosis : Official Journal of the European Cystic Fibrosis Society 2015;14(4):507‐14. [CENTRAL: 1038490; CFGD Register: PI262c ; CRS: 5500131000000327; JID:: 101128966; PUBMED: 25592656] - PubMed
-
- Elborn JS, Geller DE, Conrad D, Aaron SD, Smyth AR, Fischer R, et al. A phase 3, open‐label, randomized trial to evaluate the safety and efficacy of levofloxacin inhalation solution (APT‐1026) versus tobramycin inhalation solution in stable cystic fibrosis patients. Journal of Cystic Fibrosis : Official Journal of the European Cystic Fibrosis Society 2015;14(4):507‐14. Online supplementary material. [CENTRAL: 1038490; CFGD Register: PI262e; CRS: 5500131000000327; JID:: 101128966; PUBMED: 25592656] - PubMed
Flume 2015a {published data only}
-
- Fischer R, Flume PA, Devanter DR, Polu K, Pecoraro M, Bhatt N, et al. Pulmonary exacerbations and changes in lung function in CF adults with P. aeruginosa treated with inhaled levofloxacin (Quinsair®) or tobramycin. Journal of Cystic Fibrosis 2016;51 Suppl 45:359. [Abstract no: 436; CENTRAL: 1262259; CFGD Register: PI283d; CRS: 5500135000001725]
-
- Flume P. Trial of aeroquin versus tobramycin inhalation solution (TIS) in cystic fibrosis (CF) patients. (www.clinicaltrials.gov) (accessed 21 Oct 2014) 2014. [CENTRAL: 1012530; CFGD Register: PI283a; CRS: 5500131000000187; 5500131000000187]
-
- Flume P, Elborn JS, Polu K, Llorens L, Pecoraro ML, Bhatt N, et al. History of pulmonary exacerbations (PEx) as a predictor of response to nebulized levofloxacin compared with nebulized tobramycin. Journal of Cystic Fibrosis : Official Journal of the European Cystic Fibrosis Society 2016;2015 Suppl 1:S61. [Abstract no: 38; CENTRAL: 1171477; CFGD Register: PI283e; CRS: 5500135000001592]
-
- Flume P, VanDevanter DR, Cohen F, Fleming R, Elborn JS. Safety profile of levofloxacin inhalation solution from 3 controlled cystic fibrosis trials [abstract]. Journal of Cystic Fibrosis : Official Journal of the European Cystic Fibrosis Society 2015;14 Suppl 1:S87. [Abstract no: 117; CENTRAL: 1077213; CFGD Register: PI240f // PI283c // PI284c; CRS: 5500135000001302]
-
- Devanter D, Flume PA, Fleming R, Elborn J. How often is pulmonary exacerbation defined by ≥4 Fuchs criteria associated with antibiotic treatment?. Pediatric Pulmonology 2014;49 Suppl 38:356. [Abstract no: 388; CENTRAL: 1012531; CFGD Register: PI283b // PI284b; CRS: 5500131000000188]
Flume 2015b {published data only}
-
- Flume PA, Clancy JP, Retsch‐Bogart GZ, Tullis E, Bresnik M, Derchak PA, et al. Aztreonam for inhalation solution (AZLI) and tobramycin inhalation solution (TIS) continuous alternating therapy (CAT) for cystic fibrosis (CF) patients with chronic pseudomonas aeruginosa (PA) infection: a randomized, double‐blind, placebo‐controlled trial. Pediatric Pulmonology 2015;50 Suppl 41:352. [Abstract no: 428; CENTRAL: 1092171; CFGD Register: PI288; CRS: 5500135000001367]
Flume 2016 {published data only}
-
- Flume P. A Phase 3, Multi‐Center, Multinational, Randomized, Double‐Blind, Placebo‐Controlled Study To Evaluate The Efficacy And Safety Of MP‐376 (Levofloxacin Inhalation Solution; Aeroquin™) In Stable Cystic Fibrosis Patients. www.clinicaltrials.gov (accessed 21 Oct 2014) 2014. [CENTRAL: 1012532; CFGD Register: PI284a; CRS: 5500131000000190; NCT01180634] - PubMed
-
- Flume P, VanDevanter DR, Cohen F, Fleming R, Elborn JS. Safety profile of levofloxacin inhalation solution from 3 controlled cystic fibrosis trials. Journal of Cystic Fibrosis : Official Journal of the European Cystic Fibrosis Society 2015;14 Suppl 1:S87. [Abstract no: 117; CENTRAL: 1077213; CFGD Register: PI240f // PI283c // PI284c; CRS: 5500135000001302]
-
- Flume PA, VanDevanter DR, Morgan EE, Dudley MN, Loutit JS, Bell SC, et al. A phase 3, multi‐center, multinational, randomized, double‐blind, placebo‐controlled study to evaluate the efficacy and safety of levofloxacin inhalation solution (APT‐1026) in stable cystic fibrosis patients. Journal of Cystic Fibrosis 2016;15(4):495‐502. [CFGD Register: PI284d] - PubMed
-
- Flume PA, VanDevanter DR, Morgan EE, Dudley MN, Loutit JS, Bell SC, et al. A phase 3, multi‐center, multinational, randomized, double‐blind, placebo‐controlled study to evaluate the efficacy and safety of levofloxacin inhalation solution (APT‐1026) in stable cystic fibrosis patients. Journal of Cystic Fibrosis 2016;15(4):495‐502. Online supplement. [CFGD Register: PI284e] - PubMed
-
- Devanter D, Flume PA, Fleming R, Elborn J. How often is pulmonary exacerbation defined by ≥4 Fuchs criteria associated with antibiotic treatment?. Pediatric Pulmonlogy 2014;49 Suppl 38:356. [Abstract no: 388; CFGD Register: PI283b // PI284b]
Frederiksen 1997 {published data only}
-
- Frederiksen B, Koch C, Høiby N. Antibiotic treatment of initial colonisation with Pseudomonas aeruginosa postpones chronic infection and prevents deterioration of pulmonary function in cystic fibrosis. Pediatric Pulmonology 1997;23(5):330‐5. - PubMed
Geller 2007 {published data only}
-
- Geller DE, Howenstine M, Conrad C, Smith J, Mulye S, Shrewsbury SB. A phase 1 study to assess the tolerability of a novel tobramycin powder for inhalation (TPI) formulation in cystic fibrosis subjects. Pediatric Pulmonology 2004;38(Suppl 27):250. [CENTRAL: 507896; CFGD Register: PI187a ; CRS: 5500100000002694]
-
- Geller DE, Konstan MW, Smith J, Noonberg SB, Conrad C. Novel tobramycin inhalation powder in cystic fibrosis subjects: Pharmacokinetics and safety. Pediatric Pulmonology 2007;42(4):307‐13. [CENTRAL: 587235; CFGD Register: PI187c ; CRS: 5500100000002919; PUBMED: 17352404] - PubMed
-
- Rodriguez CA, Shrewsbury SB, Potter SN, Nardella P, Geller DE. Single dose pharmacokinetics of tobramycin after administration of a novel dry powder formulation (TPI) in subjects with cystic fibrosis (cf). Pediatric Pulmonology 2004;38 Suppl 27:250. [CENTRAL: 526254; CFGD Register: PI187b ; CRS: 5500100000002754]
Geller 2011 {published data only}
-
- Conrad D, Flume P, Sindel L, Andrews S, Morgan L, Loutit J, et al. Phase 2b study of inhaled MP‐376 (Aeroquin, levofloxacin inhalation solution) in stable cystic fibrosis (CF) patients with chronic Pseudomonas Aeruginosa (PA) lung infection. American Journal of Respiratory and Critical Care Medicine 2010;181(Meeting Abstracts). [CENTRAL: 1031676; CFGD Register: PI240g; CRS: 5500050000000360; EMBASE: 70839804]
-
- Flume P, Geller DE, Sindel L, Staab D, Fischer R, Riethmuller J, et al. Effects of inhaled MP‐376 (aeroquin, levofloxacin inhalation solution) on lung function in stable cystic fibrosis (CF) patients with chronic Pseudomonas aeruginosa (PA) lung infection. Journal of Cystic Fibrosis 2010;9 Suppl 1:S23. [Abstract no: 86; CENTRAL: 774683; CFGD Register: PI240a; CRS: 5500100000003504]
-
- Flume P, VanDevanter DR, Cohen F, Fleming R, Elborn JS. Safety profile of levofloxacin inhalation solution from 3 controlled cystic fibrosis trials. Journal of Cystic Fibrosis : Official Journal of the European Cystic Fibrosis Society 2015;14 Suppl 1:S87. [Abstract no: 117; CENTRAL: 1077213; CFGD Register: PI240f // PI283c // PI284c; CRS: 5500135000001302]
-
- Flume PA, Geller DE, Loutit JS, Dudly MN, Conrad D, Mpex 204Sgroup. Effects of inhaled MP‐376 (Aeroquinâ„¢ levofloxacin inhalation solution) on cystic fibrosis patients with both Staphylococcus aureus (SA) and Pseudomonas aeruginosa (PA) lung infection. Journal of Cystic Fibrosis 2011;10 Suppl 1:S22. [Abstract no: 87; CENTRAL: 849020; CFGD Register: PI240b; CRS: 5500100000010582]
-
- Geller DE, Flume PA, Staab D, Fischer R, Loutit JS, Conrad DJ. Levofloxacin inhalation solution (MP‐376) in patients with cystic fibrosis with Pseudomonas aeruginosa. American Journal of Respiratory and Critical Care Medicine 2011;183(11):1510‐6. [CENTRAL: 800852; CFGD Register: PI240d; CRS: 5500100000010625] - PubMed
Gibson 2007 {published data only}
-
- Gibson RL, Emerson J, Mayer‐Hamblett N, Burns JL, McNamara S, Accurso FJ, et al. Duration of treatment effect after tobramycin solution for inhalation in young children with cystic fibrosis. Pediatric Pulmonology 2007;42(7):610‐23. - PubMed
Goss 2009 {published data only}
-
- Clancy JP, Dupont L, Konstan MW, Billings J, Fustik S, Goss CH, et al. Online supplementary data to "Phase II studies of nebulised Arikace in CF patients with Pseudomonas aeruginosa infection" [online. Thorax 2013;68(9):818‐25 online. [CFGD Register: PI222d // PI207f; CRS: 5500135000000003] - PMC - PubMed
-
- Clancy JP, Minic P, Dupont L, Goss CH, Quittner AL, Lymp JF, et al. Full analysis of data from two phase II blinded & placebo‐controlled studies of nebulized liposomal amikacin for inhalation (Arikace) in the treatment of CF patients with Pseudomonas aeruginosa lung infection. Pediatric Pulmonology 2010;45 Suppl 33:299. [Abstract no: 227; CFGD Register: PI222b //PI207d]
-
- Dupont LJ, Clancy JP, Minic P, Goss CH, Fustic S, Mazurek H, et al. Evaluation of two phase II blinded and placebo‐controlled studies of nebulized liposomal amikacin (Arikace") in the treatment of cystic fibrosis patients with Pseudomonas aeruginosa lung infection. American Journal of Respiratory and Critical Care Medicine 2010;181(Meeting abstracts). [Abstract no: A1836; CFGD Register: PI222e // PI207g]
-
- Goss CH, Clancy JP, Nick JA, Billings J, Rubenstein RC, Young KR, et al. A phase 2 blinded and placebo‐controlled study of nebulized liposomal amikacin (arikace) in the treatment of CF patients with Pseudomonas aeruginosa lung infection. Pediatric Pulmonology 2009;44(S32):295. [CFGD CF Register: PI222a]
Griese 2002 {published data only}
-
- Griese M, Mueller I, Reinhardt D. Eradication of initial Pseudomonas aeruginosa colonisation in patients with cystic fibrosis. European Journal of Medical Research 2002;7(2):79‐80. - PubMed
Heinzl 2002 {published data only}
-
- Heinzl B, Eber E, Oberwaldner B, Haas G, Zach M. Effects of inhaled gentamicin prophylaxis on acquisition of Pseudomonas aeruginosa in children with cystic fibrosis: a pilot study. Pediatric Pulmonology 2002;33(1):32‐7. - PubMed
Kenny 2009 {published data only}
-
- Kenny S, Hall V, Goldsmith C, Moore J, Rendall JC, Elborn JS. Eradication of Pseudomonas aeruginosa in adults with CF. Journal of Cystic Fibrosis 2009;8 Suppl 2:S39. [Abstract no: 158; MEDLINE: ]
Konstan 2010 {published data only}
-
- Konstan MW, Geller DE, Brockhaus F, Zhang J, Angyalosi G. Tobramycin inhalation powder is effective and safe in the treatment of chronic pulmonary Pseudomonas aeruginosa (Pa) infection in patients with cystic fibrosis. American Journal of Respiratory and Critical Care Medicine 2009;179. [Abstract no: A1186; CENTRAL: 744126; CFGD Register: PI227a; CRS: 5500100000003455]
-
- Konstan MW, Geller DE, Minic P, Brockhaus F, Zhang J, Angyalosi G. Effective treatment of chronic Pseudomonas aeruginosa (Pa) infection with tobramycin inhalation powder in CF patients. Journal of Cystic Fibrosis 2009;8 Suppl 2:S27. [Abstract no: 105; CENTRAL: 744127; CFGD Register: PI227b; CRS: 5500100000003456]
-
- McColley S, Rietschel E, Brockhaus F, Angyalosi G, Higgins M. Safety of inhaled tobramycin in patients with cystic fibrosis. Pediatric Pulmonology 2011;46 Suppl 34:344. [Abstract no: 365; CENTRAL: 848919; CFGD Register: PI227d // PI239h; CRS: 5500100000010634]
-
- Novartis (NCT00125346). Tobramycin Inhalation Powder (TIP) in Cystic Fibrosis Subjects (EVOLVE). Http://clinicaltrials.gov/ct2/show/NCT00125346 2010. [CENTRAL: 867467; CFGD Register: PI227c ; CRS: 5500100000006168]
Konstan 2011 {published data only}
-
- Chiron R, Geller DE, Angyalosi G, Debonnett L, Yadao A, Bader G, et al. Tobramycin powder for inhalation is effective in advanced stage CF lung disease: the EAGER trial. Journal of Cystic Fibrosis 2014;13 Suppl 2:S57. [Abstract no: 42; CENTRAL: 996576; CFGD Register: PI239k; CRS: 5500129000000011]
-
- Geller DE, Flume PA, Brockhaus F, Zhang J, Angyalosi G, He E, et al. Treatment convenience and satisfaction of tobramycin inhalation powder (TIP) versus TOBI in cystic fibrosis (CF) patients. Journal of Cystic Fibrosis 2010;9 Suppl 1:S22. [Abstract no: 82; CENTRAL: 776791; CFGD Register: PI239b; CRS: 5500100000003511]
-
- Geller DE, Flume PA, Konstan M, Angyalosi G, Higgins M. Microbiological and clinical response to tobramycin inhalation powder (TIPâ„¢) in cystic fibrosis patients with chronic Pseudomonas aeruginosa (Pa) infection [abstract]. Journal of Cystic Fibrosis 2011;10 Suppl 1:S21. [Abstract no: 82; CENTRAL: 848918; CFGD Register: PI239g; CRS: 5500100000010633]
-
- Geller DE, Nasr SZ, Piggott S, He E, Angyalosi G, Higgins M. Tobramycin inhalation powder in cystic fibrosis patients: response by age group. Respiratory Care 2014;59(3):388‐98. [CFGD Register: PI239l; CRS: 5500135000000283; PUBMED: 23983274] - PubMed
-
- Konstan M, Flume PA, Brockhaus F, Angyalosi G, He, E, et al. Safety and efficacy of tobramycin inhalation powder (TIP) in treating CF patients infected with Pseudomonas aeruginosa (Pa). Journal of Cystic Fibrosis 2010;9 Suppl 1:S22. [Abstract no: 82; CENTRAL: 776166; CFGD Register: PI239a; CRS: 5500100000003510]
Konstan 2015 {published data only}
-
- Bilton D, Pressler T, Fajac I, Clancy JP, Minic P, Cipolli M, et al. Analysis of long‐term liposomal amikacin for inhalation in patients with cystic fibrosis and chronic infection from pseudomonas aeruginosa. Pediatric Pulmonology 2014;49 Suppl 38:317‐8. [Abstract no: 284; CENTRAL: 1012524; CFGD Register: PI280a; CRS: 5500131000000176]
-
- Bilton D, Pressler T, Fajac I, Clancy JP, Minic P, Cipolli M, et al. Analysis of long‐term use of liposomal amikacin for inhalation (LAI) in patients with cystic fibrosis (CF) who have chronic infection from Pseudomonas aeruginosa. Journal of Cystic Fibrosis : Official Journal of the European Cystic Fibrosis Society 2015;14 Suppl 1:S85. [Abstract no: 112; CENTRAL: 1099087; CFGD Register: PI280b; CRS: 5500135000001303]
-
- Konstan M, Fajac I, Pressler T, Clancy JP, Sands D, Minic P, et al. Long‐term study of liposomal amikacin for inhalation (LAI) in patients with cystic fibrosis (CF) and chronic pseudomonas aeruginosa infection [abstract]. Pediatric Pulmonology 2015;50 Suppl 41:270, Abstract no: 212. [CENTRAL: 1092191; CFGD Register: PI280c; CRS: 5500135000001380]
Latzin 2008 {published data only}
-
- Latzin P, Fehling M, Bauernfeind A, Reinhardt D, Kappler M, Griese M. Efficacy and safety of intravenous meropenem and tobramycin versus ceftazidime and tobramycin in cystic fibrosis. Journal of Cystic Fibrosis 2008;7(2):142‐6. [CFGD Register: PI209] - PubMed
Lenoir 2007 {published data only}
-
- Lenoir G, Antypkin YG, Miano A, Moretti P, Zanda M, Varoli G, et al. Efficacy, safety, and local pharmacokinetics of highly concentrated nebulized tobramycin in patients with cystic fibrosis colonized with Pseudomonas aeruginosa. Paediatric Drugs 2007;9 Suppl:11‐20. [CFGD Register: PI196c] - PubMed
-
- Lenoir G, Aryayev N, Varoli G, Monici Preti P. Aerosolized tobramycin in the treatment of patients with cystic fibrosis and pseudomonas aeruginosa infection. Journal of Cystic Fibrosis 2006;5 Suppl 1:S42. [CFGD Register: PI196b]
-
- Lenoir G, Aryayev N, Varoli G, Monici Preti P. Highly concentrated aerosolized tobramycin in the treatment of patients with cystic fibrosis and Pseudomonas aeruginosa infection. European Respiratory Journal 2005;26 Suppl 49:620s. [CFGD Register: PI196a]
Littlewood 1985 {published data only}
-
- Littlewood JM, Miller MG, Ghoneim AT, Ramsden CH. Nebulised colomycin for early pseudomonal colonisation in cystic fibrosis [letter]. Lancet 1985;1(8433):865. - PubMed
Mainz 2014 {published data only}
-
- Mainz JG, Schadlich K, Schien C, Michl R, Schelhorn‐Neise P, Koitschev A, et al. Sinonasal inhalation of tobramycin vibrating aerosol in cystic fibrosis patients with upper airway Pseudomonas aeruginosa colonization: results of a randomized, double‐blind, placebo‐controlled pilot study. Drug Design, Development and Therapy 2014;8:209‐17. [CENTRAL: 981442; CFGD Register: PI248b ; CRS: 5500125000000721; PUBMED: 24596456] - PMC - PubMed
-
- Mainz JG, Schien C, Schadlich K, Pfister W, Schelhorn‐Neise P, Koitschev A, et al. Sinonasal inhalation of tobramycin in cystic fibrosis patients with P. aeruginosa colonization of the upper airways ‐ results of a multicentric placebo‐controlled pilot study. Journal of Cystic Fibrosis 2011;10 Suppl 1:S21. [Abstract no: 83; CENTRAL: 848866; CFGD Register: PI248a ; CRS: 5500100000010543]
-
- Mainz JG, Schiller I, Ritschel C, Mentzel HJ, Riethmüller J, Koitschev A, et al. Sinonasal inhalation of dornase alfa in CF: A double‐blind placebo‐controlled cross‐over pilot trial. Auris, Nasus, Larynx 2011;38(2):220‐7. [CENTRAL: 781345; CFGD Register: PI248c; CRS: 5500050000000201; PUBMED: 21030168] - PubMed
Mazurek 2012 {published data only}
-
- Mazurek H, Chiron R, Pelikan L, Geidel C, Bolbas K, Antipkin Y, et al. Comparison of two inhaled tobramycin solutions in cystic fibrosis patients with chronic pseudomonas aeruginosa infection: results in different age subgroups. Journal of Cystic Fibrosis 2011;10 Suppl 1:S28. [Abstract no: 111; CENTRAL: 848929; CFGD Register: PI249b; CRS: 5500100000010714]
-
- Mazurek H, Chiron R, Varoli G, Santoro D, Cicirello H, Antipkin Y. Efficacy on lung function and safety of multiple courses of tobramycin 300mg/4 ml nebuliser solution (Bramitob) in patients with cystic fibrosis and chronic pseudomonas aeruginosa infection: results from a 48‐week extension phase. Journal of Cystic Fibrosis 2012;11 Suppl1:S74. [Abstract no: 69; CENTRAL: 867264; CFGD Register: PI249c; CRS: 5500100000011290]
-
- Mazurek H, Lenoir G, Pelikan L, Geidel C, Bolbas K, Antipkin Y, et al. Head‐to‐head comparison of two inhaled tobramycin solutions in cystic fibrosis (CF) patients with chronic pseudomonas aeruginosa (Pa) infection. Journal of Cystic Fibrosis 2011;10 Suppl 1:S28. [Abstract no: 110; CENTRAL: 848928; CFGD Register: PI249a; CRS: 5500100000010713]
Oermann 2009 {published data only}
-
- Oermann CM, McCoy KS, Retsch‐Bogart GZ, Gibson R, McKevitt M, Montgomery B. Antibiotic susceptibility in Pseudomonas Aeruginosa (PA) isolates following exposure to aztreonam for inhalation solution (AZLI) in patients with cystic fibrosis. Pediatric Pulmonology 2009;44(S32):309. [Abstract no: 278; CFGD CF Register: PI220a]
-
- Oermann CM, McCoy KS, Retsch‐Bogart GZ, Gibson R, McKevitt M, Montgomery B. Effect of repeated exposure to aztreonam for inhalation solution (AZLI) therapy on cystic fibrosis respiratory pathogens. Pediatric Pulmonology 2009;44(Suppl 32):335. [Abstract no: 353; CFGD Register: PI220d]
-
- Oermann CM, McCoy KS, Retsch‐Bogart GZ, Gibson RL, Montgomery AB. Effect of multiple courses of Aztreonam Lysine for inhalation (AZLI) on FEV1 and weight in patients with cystic fibrosis (CF) and Pseudomonas aeruginosa (PA): analysis of 18 month data from CP‐AI‐006. Journal of Cystic Fibrosis 2009;8 Suppl 2:S28. [Abstract no: 107; CFGD CF Register: PI220c]
-
- Oermann CM, McCoy KS, Retsch‐Bogart GZ, Gibson RL, Quittner AL, Montgomery AB. Adherence over multiple courses of Aztreonam for inhalation (AZLI): effect on disease‐ related endpoints in patients with cystic fibrosis (CF) and Pseudomonas aeruginosa (PA). Journal of Cystic Fibrosis 2009;8 Suppl 2:S28. [Abstract no: 109; CFGD CF Register: PI220b]
Postnikov 2000 {published data only}
-
- Postnikov SS, Semykin SYU, Kapranov NI, Perederko LV, Polikarpova SV, Khamidullina KF. Evaluation of pefloxacin efficacy and tolerability in the treatment and prophylaxis of severe infections at the children with mucoviscidosis and aplastic anaemia. Antibiotiki i Khimioterapiia 2000;45(8):25‐30. [CFGD Register: PI171] - PubMed
Postnikov 2007 {published data only}
-
- Postnikov SS, Semykin SY, Polikarpova SV, Dubovik LG, Gracheva LA, Sagatelyan. A prospective trial on the efficacy and tolerability of twice‐daily dosing (TDD) versus once‐daily dosing (ODD) amikacin in cystic fibrosis patients. Journal of Cystic Fibrosis 2007;6 Suppl 1:S34. [CFGD CF Register: PI204]
Prayle 2013 {published data only}
-
- Prayle A, Jain K, Watson A, Smyth AR. Are morning doses of intravenous tobramycin less nephrotoxic than evening? Evidence from urinary biomarkers in the critic study. Pediatric Pulmonology 2013;48 Suppl 36:299. [Abstract no: 261; CENTRAL: 980338; CFGD Register: CO55 ; CRS: 5500125000000420]
Ramsey 1999 {published data only}
-
- Birnbaum HG, Greenberg P, Finkelstein S, Berndt E, Otto KL, Montgomery AB, et al. Economic analysis of hospitalization and home IV anti‐pseudomonal antibiotic use in CF patients on tobramycin solution for inhalation (TOBI®). Pediatric Pulmonology 1998;Suppl 17:273. [CENTRAL: 792738; CRS: 5500100000003553]
-
- Bowman CM. The long‐term use of inhaled tobramycin in patients with cystic fibrosis. Journal of Cystic Fibrosis 2002;1 Suppl 2(Suppl 2):S194‐8. [CENTRAL: 451888; CFGD Register: PI120cc; CRS: 5500100000002389] - PubMed
-
- Burns JL, Dalfsen JM, Shawar RM, Otto KL, Garber RL, Quan JM, et al. Effect of chronic intermittent administration of inhaled tobramycin on respiratory microbial flora in patients with cystic fibrosis. Journal of Infectious Diseases 1999;179(5):1190‐6. [CENTRAL: 161606; CRS: 5500100000000895; PUBMED: 10191222] - PubMed
-
- Casey S, Ramsey B, Borowitz D. Nutritional benefits of chronic intermittent Pseudomonas aeruginosa suppression with tobramycin solution for inhalation in adolescents. 13th International Cystic Fibrosis Congress; 2000 June 4‐8; Stockholm, Sweden. 2000:172. [CENTRAL: 302945; CRS: 5500100000001685]
-
- Enger C, Rothman K, Kylstra JW. Mortality rates during 2 years of treatment with intermittent inhaled tobramycin (TOBI) in CF. Pediatric Pulmonology 1999;Suppl 19:339‐40. [CENTRAL: 291292; CRS: 5500100000001353]
Ratjen 2001a {published data only}
-
- Ratjen F, Döring G, Nikolaizik WH. Effect of inhaled tobramycin on early Pseudomonas aeruginosa colonisation in patients with cystic fibrosis. Lancet 2001;358(9286):983‐4. - PubMed
Retsch‐Bogart 2008 {published data only}
-
- Burns JL, Stapp J, Lofland D, AIPhase 2SG. Microbiology results from a phase 2 clinical study of aztreonam lysinate for inhalation (AI): a new inhaled antibiotic to treat CF patients with Pseudomonas aeruginosa (PA). Journal of Cystic Fibrosis 2005;4 Suppl:S55. [CENTRAL: 548338; CFGD Register: PI211a ; CRS: 5500100000002786]
-
- Retsch‐Bogart GZ, Burns JL, Otto KL, Liou TG, McCoy K, Oermann C, et al. A phase 2 study of aztreonam lysine for inhalation to treat patients with cystic fibrosis and Pseudomonas aeruginosa infection. Pediatric Pulmonology 2008;43(1):47‐58. [CENTRAL: 628521; CFGD Register: PI211c; CRS: 5500100000003193; PUBMED: 18041081] - PubMed
-
- Retsch‐Bogart GZ, Gibson RL, AIPhase 2SG. A phase 2 study of aztreonam lysinate for inhalation to treat cystic fibrosis patients with Pseudomonas aeruginosa infection. American Thoracic Society International Conference; 2005 May 20‐25; San Diego, USA. 2005:A576. [CENTRAL: 592963; CFGD Register: PI211b ; CRS: 5500100000002930]
-
- Retsch‐Bogart GZ, McCoy KS, Gibson RL, Oermann CM. Sustained improvement in pulmonary function following a 28‐day course of 75mg azli tid therapy. Pediatric Pulmonology 2008;43 Suppl 31:320. [CENTRAL: 675920; CFGD Register: PI211d // PI213b; CRS: 5500100000003286]
Retsch‐Bogart 2009 {published data only}
-
- McCoy K, Retsch‐Bogart G, Gibson RL, Oermann C, Braff M, Montgomery AB. Investigation of susceptibility breakpoints for inhaled antibiotic therapies in cystic fibrosis. Pediatric Pulmonology 2010;45 Suppl 33:341. [Abstract no: 340; CENTRAL: 848913; CFGD Register: PI212g // PI213i ; CRS: 5500100000010623]
-
- McCoy KS, Retsch‐Boagrt GZ, Gibson RL, Oermann CM, McKevitt M, Montgomery AB. Efficacy of Aztreonam Lysine for inhalation (AZLI) in patients with cystic fibrosis and drug resistant P. aeruginosa (DRPA). Journal of Cystic Fibrosis 2009;8 Suppl 2:S28. [CENTRAL: 744120; CFGD Register: PI212e // PI213e ; CRS: 5500100000003449]
-
- McCoy KS, Retsch‐Bogart GZ, Gibson R, Oermann C, Braff MH, Montgomery AB. Relevance of established susceptibility breakpoints to clinical efficacy of inhaled antibiotic therapies in cystic fibrosis. Pediatric Pulmonology 2008;43 Suppl 31:351. [CENTRAL: 677646; CFGD Register: PI212b // PI213c; CRS: 5500100000003289]
-
- Plosker GL. Aztreonam lysine for inhalation solution: in cystic fibrosis. Drugs 2010;70(14):1843‐55. [CENTRAL: 848912; CFGD Register: PI212f // PI213h ; CRS: 5500100000010622] - PubMed
-
- Quittner AL, Henig NR, Lewis S, Derchak PA, McCoy KS, Oermann CM, et al. Effects of chronic intermittent aztreonam for inhalation solution (AZLI) on health‐related quality of life (HRQOL) in persons with cystic fibrosis (CF) and P. aeruginosa. Pediatric Pulmonology 2011;46 Suppl 34:299. [Abstract no: 240; CENTRAL: 867260; CFGD Register: PI213j // PI220f; CRS: 5500100000011261]
Rietschel 2009 {published data only}
-
- Rietschel E, Posselt HG, Heuer HE, Merkel N, Staab D. Pharmacokinetics of tobramycin (TOBI) after 4 and 8 weeks of continuous once daily or twice daily inhalation. Journal of Cystic Fibrosis 2009;8 Suppl 2:S27. [Abstract no: 106; CENTRAL: 795021; CFGD Register: PI232a; CRS: 5500100000003587]
-
- Rietschel E, Staab D, Merkel N, Konigsbruggen S, Posselt H. Pharmacokinetics of continuous treatment with O.D. or B.I.D inhalation of tobramycin (TOBI™) via Pari Eflow™ Rapid. Pediatric Pulmonology 2010;45 Suppl 33:320. [Abstract no: 283; CENTRAL: 849031; CFGD Register: PI232c; CRS: 5500100000010629]
-
- Rietschel E, Staab D, Konigsbruggen S, Merkel N, Heuer HE, Posselt HG. Pharmacokinetics of continuous treatment with o.d. or b.i.d. inhalation of tobramycin (TOBITM). Journal of Cystic Fibrosis 2010;9 Suppl 1:S23. [Abstract no: 106; CENTRAL: 795018; CFGD Register: PI232b; CRS: 5500100000003586]
Ruddy 2013 {published data only}
-
- Ruddy J, Emerson J, Moss R, Genatossio A, McNamara S, Burns JL, et al. Sputum tobramycin concentrations in cystic fibrosis patients with repeated administration of inhaled tobramycin. Journal of Aerosol Medicine and Pulmonary Drug Delivery 2013;26(2):69‐75. [5K23RR15529/RR/NCRR: NIH HHS/United States; CENTRAL: 880221; CFGD Register: PI277; CRS: 5500127000000010; GR:: 1UL1RR025744/RR/NCRR NIH HHS/United States; JID:: 101475057; K23: RR015529/RR/NCRR NIH HHS/United States; OID: [Other ID]: NLM: PMC3621259; PMCID:: PMC3621259; PUBMED: 22620494; UL1: TR000423/TR/NCATS NIH HHS/United States] - PMC - PubMed
Schaad 1997 {published data only}
-
- Schaad UB, Wedgwood J, Ruedeberg A, Kraemer R, Hampel B. Ciprofloxacin as antipseudomonal treatment in patients with cystic fibrosis. Pediatric Infectious Diseases Journal 1997;16(1):106‐11. [CFGD Register: PI116] - PubMed
Schelstraete 2010 {published data only}
-
- Schelstraete P, Deschaght P, Daele S, Haerynck F, Simaey L. Genotype based evaluation of eradication treatment of new P. aeruginosa infections in CF patients. Journal of Cystic Fibrosis 2009;8 Suppl 2:S39. [Abstract no: 156; CFGD CF Register: PI228; MEDLINE: ]
-
- Schelstraete P, Deschaght P, Simaey L, Daele S, Haerynck F, Vaneechoutte M, et al. Genotype based evaluation of Pseudomonas aeruginosa eradication treatment success in cystic fibrosis patients. Journal of Cystic Fibrosis 2010;9(2):99‐103. [CFGD Register: PI228] - PubMed
Schuster 2013 {published data only}
-
- Goldman M, Schuster A, Halliburn C, Döring G, The FSG. A randomised, open label phase 3 study to evaluate the efficacy and safety of a dry powder formulation of inhaled colistimethate sodium (Colobreathe®) versus tobramycin nebuliser solution (TNS) in cystic fibrosis subjects with chronic Pseudomonas aeruginosa lung infection. Journal of Cystic Fibrosis 2012;11, Suppl 1:S12. [Abstract no: WS5.5; CENTRAL: 848917; CFGD Register: PI214b ; CRS: 5500100000010630]
-
- Goldman MH, Pitt T. Lack of emergence of antimicrobial resistance of Pseudomonas Aeruginosa after six months inhalation of dry powder colistimethate. Pediatric Pulmonology 2008;43 Suppl 31:331. [CFGD Register: PI214a]
-
- Goldman MH, Shuster A, Haliburn C, Doring G. A randomised, open label phase 3 study to evaluate the efficacy and safety of a dry powder formulation of colistimethate sodium (Colobreathe®) versus tobramycin nebuliser solution (TNS) in cystic fibrosis subjects with chronic Pseudomonas aeruginosa lung infection. Pediatric Pulmonology 2012;47(S35):353. [Abstract no: 363; CENTRAL: 849030; CFGD Register: PI214c ; CRS: 5500100000010631]
-
- Goldman MH, Werner T, Schuster A. Does persistence with inhaled dry powder antibiotic treatment improve tolerability?. Pediatric Pulmonology 2013;48 Suppl 36:347. [Abstract no: 389; CENTRAL: 921669; CFGD Register: PI214f; CRS: 5500125000000396]
-
- Schuster A, Haliburn C, Doring G, Goldman MH, FSG. Online Data Supplement to 'Safety, efficacy and convenience of colistimethate sodium dry powder for inhalation (Colobreathe DPI) in patients with cystic fibrosis: a randomised' [online] study. Thorax 2013;68(4):344‐350 Online. [CENTRAL: 867327; CFGD Register: PI214e; CRS: 5500100000011299] - PMC - PubMed
Stass 2013 {published data only}
-
- Stass H, Delesen H, Nagelschmitz J, Staab D. Safety and pharmacokinetics of ciprofloxacin dry powder for inhalation in cystic fibrosis: a Phase I, randomized, single‐dose, dose‐escalation study. Journal of Aerosol Medicine and Pulmonary Drug Delivery 2015;28(2):106‐15. [CENTRAL: 1037796; CFGD Register: PI215c ; CRS: 5500131000000317; JID:: 101475057; PUBMED: 25050456] - PubMed
-
- Stass H, Ludwig M, Nagelschmitz J, Stabb D. Safety and pharmacokinetics of inhaled dry powder ciprofloxacin after single and multiple inhalations in patients with cystic fibrosis. Paediatric Pulmomology 2008;43 Suppl 31:300. [CFGD Register: PI215a]
-
- Stass H, Weimann B, Nagelschmitz J, Rolinck‐Werninghaus C, Staab D. Tolerability and pharmacokinetic properties of ciprofloxacin dry powder for inhalation in patients with cystic fibrosis: a phase I, randomized, dose‐escalation study. Clinical Therapeutics 2013;35(10):1571‐81. [CFGD Register: PI215b; CRS: 5500127000000012; JID:: 7706726; PUBMED: 24054830] - PubMed
Steinkamp 1989 {published data only}
Steinkamp 2007 {published data only}
-
- Steinkamp G, Schmitt‐Grohe S, Doring G, Stabb D, Schubert R, Zielen S. Once weekly azithromycin in cystic fibrosis: a double‐blind, randomised trial in patients with chronic Pseudomonas aeruginosa infection. Pediatric Pulmonology 2007;42(S30):300. [CFGD Register: MA19b]
-
- Steinkamp G, Schmitt‐Grohe S, Doring G, Worlitzsch D, Staab D, Schubert R, et al. Clinical and immunomodulatory effects of once weekly azithromycin treatment in cystic fibrosis patients chronically infected with Pseudomonas aeruginosa. Journal of Cystic Fibrosis 2006;5 Suppl 1:S25. [CFGD Register: MA19a]
Stockmann 2015 {published data only}
-
- Geller DE, Flume P, Schwab R, Fornos P, Conrad DJ, Morgan E, et al. A phase 1 safety, tolerability and pharmacokinetic (PK) study of MP‐376 (levofloxacin solution for inhalation) in stable cystic fibrosis (CF) patients. Paediatric Pulmonology 2008;43 Suppl 31:315. [Abstract no: 321; CFGD Register: PI210b]
-
- Griffith DC, Hansen C, Pressler T, Balchen T, Jensen TJ, Geller DE, et al. Single‐dose pharmacokinetics of aerosol MP‐376 (levofloxacin solution for inhalation) in cystic fibrosis patients: PK‐PD implications. Journal of Cystic Fibrosis 2008;7(Suppl 2):S26. [CFGD Register: PI210a]
-
- Kearns GL, Rubino CM, Griffith DC, Geller DE, Forrest A, Bhavnani SM, et al. Levofloxacin pharmacokinetics (PK) after administration of MP‐376 (Levofloxacin inhalation solution; Aeroquin) in children with cystic fibrosis [abstract]. Journal of Cystic Fibrosis : Official Journal of the European Cystic Fibrosis Society 2011;10 Suppl 1:S23, Abstract no: 88. [CENTRAL: 1053535; CFGD Register: PI210d; CRS: 5500133000000015]
-
- Stockmann C, Hillyard B, Ampofo K, Spigarelli MG, Sherwin CM. Levofloxacin inhalation solution for the treatment of chronic Pseudomonas aeruginosa infection among patients with cystic fibrosis. Expert Review of Respiratory Medicine 2015;9(1):13‐22. [CENTRAL: 1053533; CFGD Register: PI210c; CRS: 5500131000000314; JID:: 101278196; PUBMED: 25417708] - PubMed
Taccetti 2005 {published data only}
-
- Taccetti G, Campana S, Festini F, Mascherini M, Doring G. Early eradication therapy against Pseudomonas aeruginosa in cystic fibrosis patients. European Respiratory Journal 2005;26(3):1‐4. - PubMed
Tramper‐Stranders 2009 {published data only}
-
- Tramper‐Stranders G, Wolfs TFW, Aalderen W, Kouwenberg J, Nagelkerke A, Ent CK. Prevention of initial P. aeruginosa infection in children with cystic fibrosis: a multi‐centre double‐blind randomised controlled trial. Journal of Cystic Fibrosis 2009;8 Suppl 2:S37. [Abstract no: 148; CFGD Register: PI231a; MEDLINE: ]
-
- Tramper‐Stranders GA, Wolfs TF, Haren Noman S, Aalderen WM, Nagelkerke AF, Nuijsink M, et al. Controlled trial of cycled antibiotic prophylaxis to prevent initial Pseudomonas aeruginosa infection in children with cystic fibrosis. Thorax 2010;65(10):915‐20. [CENTRAL: 761942; CFGD Register: PI231b; CRS: 5500125000000359; PUBMED: 20729233] - PubMed
Trapnell 2012 {published data only}
-
- McColley SA, Trapnell B, Kissner D, McKevitt M, Montgomery B, Rosen J, et al. Fosfomycin/tobramycin for inhalation (FTI): microbiological results of a phase 2 placebo‐controlled trial in patients with cystic fibrosis and pseudomonas aeruginosa [abstract]. Pediatric Pulmonology 2010;45 Suppl 33:338, Abstract no: 334. [CENTRAL: 848865; CFGD Register: PI247c; CRS: 5500100000010542]
-
- Trapnell BC, Kissner D, Montgomery AB, Newcomb T, Geller D. Fosfomycin/tobramycin for inhalation FTI): safety results of a phase 2 placebo‐controlled trial in patients with cystic fibrosis and pseudomonas aeruginosa. Pediatric Pulmonology 2010;45 Suppl 33:302. [Abstract no: 234; CENTRAL: 848864; CFGD Register: PI247b; CRS: 5500100000010541]
-
- Trapnell BC, McColley SA, Kissner DG, Rolfe MW, Rosen JM, McKevitt M, et al. Fosfomycin/tobramycin for inhalation in patients with cystic fibrosis with pseudomonas airway infection. American Journal of Respiratory and Critical Care Medicine 2012;185(2):171‐8. [CENTRAL: 814492; CFGD Register: PI247d; CRS: 5500100000010632] - PMC - PubMed
-
- Trapnell BC, Rolfe M, McColley S, Montgomery AB, Moorehead L, Geller D. Fosfomycon/tobramycin for inhalation (FTI): efficacy results of a phase 2 placebo‐controlled trial in patients with cystic fibrosis and pseudomonas aeruginosa. Pediatric Pulmonology 2010;45 Suppl 33:302. [Abstract no: 233; CENTRAL: 848927; CFGD Register: PI247a; CRS: 5500100000010712]
Vazquez 1993 {published data only}
-
- Vazquez C, Municio M, Corera M, Gaztelurrutia L, Sojo A, Vitoria JC. Early treatment of Pseudomonas aeruginosa colonisation in cystic fibrosis. Acta Paediatrica Scandanavia 1993;82(3):308‐9. - PubMed
Wainwright 2011a {published data only}
-
- Byrnes CA, Vidmar S, Cheney JL, Carlin JB, Armstrong DS, Cooper PJ, et al. Prospective evaluation of respiratory exacerbations in children with cystic fibrosis from newborn screening to 5 years of age. Thorax 2013;68(7):643‐51. [CENTRAL: 904924; CFGD Register: PE167i; CRS: 5500125000000477; PUBMED: 23345574] - PMC - PubMed
-
- Cheney J, Vidmar S, Grimwood K, Carlin JB, Wainwright C, on behalfofACFBALSG. Interim outcomes of a Pseudomonas aeruginosa (Pa) eradication protocol in young children in the Australian Cystic Fibrosis Bronchoalveolar Lavage (ACFBAL) Study. Journal of Cystic Fibrosis 2009;8(Suppl 2):S39. [Abstract no: 157; CFGD Register: PE167c]
-
- Cheney J, Wainwright C, ACFBAL SG. Trials, tribulations and triumphs of a cystic fibrosis study ‐ a behind the scenes look at the workings of an international multi‐centre study. Journal of Cystic Fibrosis 2010;9 Suppl 1:S118. [Abstract no: 452; CENTRAL: 790042; CFGD Register: PE167g; CRS: 5500125000000092]
-
- Kidd TJ, Ramsay KA, Vidmar S, Carlin JB, Bell SC, Wainwright CE, et al. Pseudomonas aeruginosa genotypes acquired by children with cystic fibrosis by age 5‐years. Journal of Cystic Fibrosis : Official Journal of the European Cystic Fibrosis Society 2015;14(3):361‐9. [CENTRAL: 1131104; CRS: 5500135000001467; PE167k; PUBMED: 25563522] - PubMed
-
- Moodie M, Lal A, Vidmar S, Armstrong DS, Byrnes CA, Carlin JB, et al. Costs of bronchoalveolar lavage‐directed therapy in the first 5 years of life for children with cystic fibrosis. Journal of Pediatrics 2014;165(3):564‐9. [CFGD Register: PE167j; CRS: 5500131000000365; JID:: 0375410; PUBMED: 24996984; SI:: ANZCTR/ACTRN12605000665639] - PubMed
Wainwright 2011b {published data only}
-
- Wainwright C, Nakamura C, Geller D, Montgomery AB. A double‐blind, multinational, randomized, placebo‐controlled trial evaluating aztreonam for inhalation solution (AZLI) in patients with cystic fibrosis (CF), mild lung disease and P. aeruginosa. Journal of Cystic Fibrosis 2010;9 Suppl 1:S22. [Abstract no: 81; CFGD Register: PI243a]
-
- Wainwright CE, Quittner AL, Geller DE, Nakamura C, Wooldridge JL, Gibson RL, et al. Aztreonam for inhalation solution (AZLI) in patients with cystic fibrosis, mild lung impairment, and P. aeruginosa. Journal of Cystic Fibrosis 2011;10(4):234‐42. [CENTRAL: 801029; CFGD Register: PI243b; CRS: 5500100000011273; PUBMED: 21441078] - PubMed
References to studies awaiting assessment
Noah 2010 {published data only}
-
- Noah T, Ivins S, Abode K, Harris W, Henry M, Leigh M. Comparison of antibiotics for early pseudomonas infection in CF: interim data analysis. Pediatric Pulmonology 2007;42(S30):332. [CFGD Register: PI205a]
-
- Noah TL, Ivins SS, Abode KA, Stewart PW, Michelson PH, Harris WT, et al. Inhaled versus systemic antibiotics and airway inflammation in children with cystic fibrosis and Pseudomonas. Pediatric Pulmonology 2010;45(3):281‐90. [CFGD Register: PI205b] - PubMed
References to ongoing studies
Ratjen 2016 {published data only}
-
- Ratjen F, Alon N, Maykut R, Liu C, Angyalosi G. TOBI® for eradication of early P. aeruginosa infection in paediatric cystic fibrosis patients: the EARLY study. Journal of Cystic Fibrosis : Official Journal of the European Cystic Fibrosis Society 2016;15 Suppl 1. [Abstract no: WS01.2; CENTRAL: 1171484; CFGD Register: PI290; Clinicaltrials.gov: NCT01082367; CRS: 5500135000001599]
TORPEDO Trial {published data only}
-
- Langton Hewer S. TORPEDO‐CF. http://www.controlled‐trials.com/ISRCTN02734162/torpedo‐cf accessed 10 October 2011. [ISRCTN02734162]
Additional references
Abman 1991
-
- Abman SH, Ogle JW, et al. Early bacteriologic, immunologic and clinical courses of young infants with cystic fibrosis identified by neonatal screening. Journal of Pediatrics 1991;119(2):211‐7. - PubMed
Anstead 2013
Armstrong 1996
-
- Armstrong D, Grimwood K, Carlin J, Carzino R, Olinsky A, Phelan P. Bronchoalveolar lavage or oropharyngeal cultures to identify lower respiratory pathogens in infants with cystic fibrosis. Pediatric Pulmonology 1996;21(5):267‐75. - PubMed
Burns 2001
-
- Burns JL, Gibson RL, McNamara S, Yim D, Emerson J, Rosenfeld M, et al. Longitudanal assessment of Pseudomonas aeruginosa in young children with cystic fibrosis. Journal of Infectious Diseases 2001;183(3):444‐52. - PubMed
Ciofu 2012
-
- Ciofu O, Mandsberg LF, Wang H, Hoiby N. Phenotypes selected during chronic lung infection in cystic fibrosis patients: implications for the treatment of Pseudomonas aeruginosa biofilm infections. FEMS Immunology and Medical Microbiology 2012;65(2):215‐25. - PubMed
Douglas 2009
-
- Douglas TA, Brennan S, Gard S, Berry L, Gangell C, Stick SM, et al. Acquisition and eradication of P. aeruginosa in young children with cystic fibrosis. European Respiratory Journal 2009;33(2):305‐11. - PubMed
Döring 2000
-
- Döring G, Conway S, Heijerman H, Hodson M, Høiby N, Smyth A, et al. Antibiotic therapy against Pseudomonas aeruginosa: a European consensus. European Respiratory Journal 2000;16(4):749‐67. - PubMed
Döring 2012
-
- Döring G, Flume P, Heijerman H, Elborn JS. Treatment of lung infection in patients with cystic fibrosis: Current and future strategies. Journal of Cystic Fibrosis 2012;11(6):461‐79. - PubMed
Emerson 2002
-
- Emerson J, Rosenfeld M, McNamara S, Ramsey B, Gibson RL. Pseudomonas aeruginosa and other predictors of mortality and morbidity in young children with cystic fibrosis. Pediatric Pulmonology 2002;34(2):91‐100. - PubMed
FitzSimmons 1993
-
- FitzSimmons SC. The changing epidemiology of cystic fibrosis. Journal of Pediatrics 1993;122(1):1‐9. - PubMed
Fitzsimmons 1996
-
- Fitzsimmons S. The Cystic Fibrosis Foundation Patient Registry Report 1996. Pediatric Pulmonology 1996;Suppl 21:267‐75.
Higgins 2003
Higgins 2011
-
- Higgins JPT, Altman DG. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook of Systematic Reviews of Interventions. Version 5.1 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hilliard 2007
Hudson 1993
-
- Hudson V, Wielinski C, Regelmann W. Prognostic implications of initial oropharyngeal bacterial flora in patients with cystic fibrosis diagnosed before the age of two years. Journal of Pediatrics 1993;122(6):854‐60. - PubMed
Iacocca 1963
-
- Iacocca VF, Sibinga M, Barbero G. Respiratory Tract Bacteriology in Cystic Fibrosis. American Journal of Disease in Childhood 1963;106(3):115‐24. - PubMed
Johansen 2015
Kerem 1990
-
- Kerem E, Corey M, Stein R, Gold R, Levison H. Risk factors for Pseudomonas aeruginosa colonisation in cystic fibrosis patients. Pediatric Infectious Disease Journal 1990;9(7):494‐8. - PubMed
Kosorok 2001
-
- Kosorok MR, Zeng L, West SE, Rock MJ, Splaingard ML, Laxova A, et al. Acceleration of lung disease in children with cystic fibrosis after Pseudomonas aeruginosa acquisition. Pediatric Pulmonology 2001;32(4):277‐87. - PubMed
Lee 2003
-
- Lee TWR, Brownlee KG, Conway SP, Denton M, Littlewood JM. Evaluation of a new definition for chronic Pseudomonas aeruginosa infection in cystic fibrosis patients. Journal of Cystic Fibrosis 2003;2(1):29‐34. - PubMed
Lee 2004
-
- Lee TW, Brownlee KG, Denton M, Littlewood JM, Conway SP. Reduction in prevalence of chronic Pseudomonas aeruginosa infection at a regional pediatric cystic fibrosis center. Pediatric Pulmonology 2004;37(2):104‐10. - PubMed
Lee 2009
-
- Lee TWR. Eradication of early Pseudomonas infection in cystic fibrosis. Chronic Respiratory Disease 2009;6(2):99‐107. - PubMed
Montori 2005
-
- Montori VM, Devereaux PJ, Adhikari NKJ, Burns KEA, Eggert CH, Briel M, et al. Randomized Trials Stopped Early for Benefit: A Systematic Review. JAMA 2005;294(17):2203‐9. - PubMed
Muhlebach 1999
-
- Muhlebach M, Stewart P, Leigh M, Noah T. Quantitation of inflammatory responses to bacteria in young cystic fibrosis and control patients. American Journal of Respiratory & Critical Care Medicine 1999;160(1):186‐91. - PubMed
Munck 2001
-
- Munck A, Bonacorsi S, Mariani‐Kurkdjian P, Lebourgeois M, Gerardin M, Brahimi N, et al. Genotypic characterisation of Pseudomonas aeruginosa strains recovered from patients with cystic fibrosis after initial and subsequent colonisation. Pediatric Pulmonology 2001;32(4):288‐92. - PubMed
Nixon 2001
-
- Nixon GM, Armstrong DS, Carzino R, Carlin JB, Olinsky A, Robertson CF, et al. Clinical outcome after early Pseudomonas aeruginosa infection in cystic fibrosis. Journal of Pediatrics 2001;138(5):699‐704. - PubMed
Pamukcu 1995
-
- Pamukcu A, Bush A, Buchdal R. Effects of Pseudomonas aeruginosa colonisation on lung function and anthropomorphic variables in children with cystic fibrosis. Pediatric Pulmonology 1995;19(1):10‐5. - PubMed
Parad 1999
Ramsey 1996
-
- Ramsey B. Drug therapy: Management of pulmonary disease in patients with cystic fibrosis. New England Journal of Medicine 1996;335(3):179‐88. - PubMed
Ratjen 2001b
-
- Ratjen F, Comes G, Paul K, Posselt H, Wagner T, Harms K. Effect of continuous antistaphylococcal therapy on the rate of P.aeruginosa acquisition in patients with cystic fibrosis. Pediatric Pulmonology 2001;31(1):13‐6. - PubMed
Rosenfeld 1999
-
- Rosenfeld M, Emerson J, Accurso F, Armstrong D, Castille R, Grimwood K, et al. Diagnostic accuracy of oropharyngeal cultures in infants and young children with cystic fibrosis. Pediatric Pulmonology 1999;28(5):321‐8. - PubMed
Rosenfeld 2001
-
- Rosenfeld MR, Gibson RL, McNamara S, Emerson J, Burns JL, Castile R, et al. Early pulmonary infection, inflammation and clinical outcomes in infants with cystic fibrosis. Pediatric Pulmonology 2001;32(5):356‐66. - PubMed
Schulz 1995
-
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in randomised controlled trials. JAMA 1995;273(5):408‐12. - PubMed
Smyth 2014
-
- Smyth AR, Bell SC, Bojcin S, Bryon M, Duff A, Flume P, et al. European Cystic Fibrosis Society Standards of Care: Best Practice Guidelines. Journal of Cystic Fibrosis 2014;13(Supplement 1):S23‐S42. - PubMed
Smyth 2017
Stutman 2002
-
- Stutman HR, Lieberman JM, Nussbaum E, Marks MI. Antibiotic prophylaxis in infants and young children with cystic fibrosis: a randomised controlled trial. Journal of Pediatrics 2002;140(3):299‐305. - PubMed
Thomassen 1984
-
- Thomassen MJ, Klinger JD, Badger SJ, Herckren BW, Stern RC. Cultures of thoracotomy specimens confirm usefulness of sputum cultures in cystic fibrosis. Journal of Pediatrics 1984;104(3):352‐6. - PubMed
UK CF Registry 2012
-
- UK CF Trust. Cystic Fibrosis Trust Patient Registry User’s Guide. Bromley: UK CF Trust, 2012.
UK CF Registry 2015
-
- UK Cystic Fibrosis Trust. UK CF Registry: Annual Data Report 2015. Bromley: Cystic Fibrosis Trust, 2016.
UK CF Trust 2004
-
- UK CF Trust Control of Infection Group. Pseudomonas aeruginosa infection in people with cystic fibrosis: suggestions for prevention and control (2nd edition). UK CF Trust 2004.
Winnie 1991
-
- Winnie GB, Cowan RG. Respiratory tract colonisation with Pseudomonas aeruginosa in cystic fibrosis: correlations between anti‐Pseudomonas aeruginosa antibody levels and pulmonary function. Pediatric Pulmonology 1991;10(2):92‐100. - PubMed
References to other published versions of this review
Langton‐Hewer 2009
Langton‐Hewer 2014
Wood 2003
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

