Roles of the PDZ-binding motif of HPV 16 E6 protein in oncogenic transformation of human cervical keratinocytes
- PMID: 28440909
- PMCID: PMC5497797
- DOI: 10.1111/cas.13264
Roles of the PDZ-binding motif of HPV 16 E6 protein in oncogenic transformation of human cervical keratinocytes
Abstract
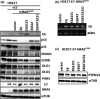
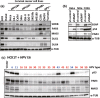
The high-risk human papillomavirus E6 proteins have been shown to interact with and lead to degradation of PDZ-domain-containing proteins through its carboxy-terminal motif. This PDZ-binding motif plays important roles in transformation of cultured cells and carcinogenesis of E6-transgenic mice. However, its biological effects on the natural host cells have not been elucidated. We have examined its roles in an in vitro carcinogenesis model for cervical cancer, in which E6 and E7 together with activated HRAS (HRASG12V ) can induce tumorigenic transformation of normal human cervical keratinocytes. In this model, E6Δ151 mutant, which is defective in binding to PDZ domains, almost lost tumorigenic ability, whereas E6SAT mutant, which is defective in p53 degradation showed activity close to wild-type E6. Interestingly, we found decreased expression of PAR3 in E6-expressing cells independently of E6AP, which has not been previously recognized. Therefore, we knocked down several PDZ-domain containing proteins including PAR3 in human cervical keratinocytes expressing E7, HRASG12V and E6Δ151 to examine whether depletion of these proteins can restore the tumorigenic ability. Single knockdown of SCRIB, MAGI1 or PAR3 significantly but partially restored the tumorigenic ability. The combinatorial knockdown of SCRIB and MAGI1 cooperatively restored the tumorigenic ability, and additional depletion of PAR3 further enhanced the tumorigenic ability surpassing that induced by wild-type E6. These data highlight the importance of the carboxy-terminal motif of the E6 protein and downregulation of PAR3 in tumorigenic transformation of human cervical keratinocytes.
Keywords: Cervical cancer; E6; PDZ domain; human cervical keratinocytes; human papillomavirus.
© 2017 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

