Cytotoxicity of Pyrazine-Based Cyclometalated (C^Npz^C)Au(III) Carbene Complexes: Impact of the Nature of the Ancillary Ligand on the Biological Properties
- PMID: 28441013
- PMCID: PMC5434479
- DOI: 10.1021/acs.inorgchem.7b00339
Cytotoxicity of Pyrazine-Based Cyclometalated (C^Npz^C)Au(III) Carbene Complexes: Impact of the Nature of the Ancillary Ligand on the Biological Properties
Abstract
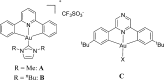
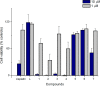
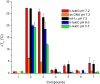
The synthesis of a series of cyclometalated gold(III) complexes supported by pyrazine-based (C^N^C)-type pincer ligands is reported, including the crystal structure of a cationic example. The compounds provide a new platform for the study of antiproliferative properties of gold(III) complexes. Seven complexes were tested: the neutral series (C^Npz^C)AuX [X = Cl (1), 6-thioguanine (4), C≡CPh (5), SPh (6)] and an ionic series that included the N-methyl complex [(C^NpzMe^C)AuCl]BF4 (7) and the N-heterocyclic carbene complexes [(C^Npz^C)AuL]+ with L = 1,3-dimethylbenzimidazol-2-ylidene (2) or 1,3,7,9-tetramethylxanthin-8-ylidene (3). Tests against human leukemia cells identified 1, 2, 3, and 4 as particularly promising, whereas protecting the noncoordinated N atom on the pyrazine ring by methylation (as in 7) reduced the cytotoxicity. Complex 2 proved to be the most effective of the entire series against the HL60 leukemia, MCF-7 breast cancer, and A549 lung cancer cell lines, with IC50 values down to submicromolar levels, associated with a lower toxicity toward healthy human lung fibroblast cells. The benzimidazolylidene complex 2 accumulated more effectively in human lung cancer cells than its caffeine-based analogue 3 and the gold(III) chloride 1. Compound 2 proved to be unaffected by glutathione under physiological conditions for periods of up to 6 days and stabilizes the DNA G-quadruplex and i-motif structures; the latter is the first such report for gold compounds. We also show the first evidence of inhibition of MDM2-p53 protein-protein interactions by a gold-based compound and identified the binding mode of the compound with MDM2 using saturation transfer difference NMR spectroscopy combined with docking calculations.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





Similar articles
-
(C^Npz^C)AuIII complexes of acyclic carbene ligands: synthesis and anticancer properties.Dalton Trans. 2017 Oct 10;46(39):13397-13408. doi: 10.1039/c7dt02804k. Dalton Trans. 2017. PMID: 28945262
-
Comparative biological evaluation and G-quadruplex interaction studies of two new families of organometallic gold(I) complexes featuring N-heterocyclic carbene and alkynyl ligands.J Inorg Biochem. 2020 Jan;202:110844. doi: 10.1016/j.jinorgbio.2019.110844. Epub 2019 Sep 11. J Inorg Biochem. 2020. PMID: 31739113
-
A Gold(III) Pincer Ligand Scaffold for the Synthesis of Binuclear and Bioconjugated Complexes: Synthesis and Anticancer Potential.Chemistry. 2018 Mar 7;24(14):3613-3622. doi: 10.1002/chem.201705902. Epub 2018 Feb 12. Chemistry. 2018. PMID: 29334159
-
New insights in Au-NHCs complexes as anticancer agents.Eur J Med Chem. 2018 Feb 25;146:709-746. doi: 10.1016/j.ejmech.2018.01.065. Epub 2018 Jan 31. Eur J Med Chem. 2018. PMID: 29407992 Review.
-
Gold(III) Complexes for Antitumor Applications: An Overview.Chemistry. 2018 Aug 14;24(46):11840-11851. doi: 10.1002/chem.201800981. Epub 2018 Jun 8. Chemistry. 2018. PMID: 29575433 Review.
Cited by
-
A Cytotoxic Bis(1,2,3-triazol-5-ylidene)carbazolide Gold(III) Complex Targets DNA by Partial Intercalation.Chemistry. 2021 Jun 4;27(32):8295-8307. doi: 10.1002/chem.202100598. Epub 2021 May 17. Chemistry. 2021. PMID: 33822431 Free PMC article.
-
Targeting Telomeres: Molecular Dynamics and Free Energy Simulation of Gold-Carbene Binding to DNA.Biophys J. 2021 Jan 5;120(1):101-108. doi: 10.1016/j.bpj.2020.11.2263. Epub 2020 Dec 5. Biophys J. 2021. PMID: 33285115 Free PMC article.
-
Small molecule targeted therapies for endometrial cancer: progress, challenges, and opportunities.RSC Med Chem. 2024 Apr 11;15(6):1828-1848. doi: 10.1039/d4md00089g. eCollection 2024 Jun 19. RSC Med Chem. 2024. PMID: 38911148 Free PMC article. Review.
-
Anticancer Gold(III) Compounds With Porphyrin or N-heterocyclic Carbene Ligands.Front Chem. 2020 Nov 6;8:587207. doi: 10.3389/fchem.2020.587207. eCollection 2020. Front Chem. 2020. PMID: 33240849 Free PMC article. Review.
-
Osmium Arene Germyl, Stannyl, Germanate, and Stannate Complexes as Anticancer Agents.ACS Omega. 2021 Jul 12;6(29):19252-19268. doi: 10.1021/acsomega.1c02665. eCollection 2021 Jul 27. ACS Omega. 2021. PMID: 34337263 Free PMC article.
References
-
- Bosl G. J.; Bajorin D. F.; Sheinfeld J.. Cancer of the Testis; DeVita V. T. J., Hellman S., Rosenberg S. A., Eds.; Lippincott Williams & Wilkins: Philadelphia, 2001.
- Watson M.; Barrett A.; Spence R.; Twelves C.. Oncology, 2nd ed.; Oxford University Press: Oxford, U.K., 2006.
-
- Rabik C. A.; Dolan M. E. Molecular mechanisms of resistance and toxicity associated with platinating agents. Cancer Treat. Rev. 2007, 33, 9–23. 10.1016/j.ctrv.2006.09.006. - DOI - PMC - PubMed
- Dilruba S.; Kalayda G. V. Platinum-based drugs: past, present and future. Cancer Chemother. Pharmacol. 2016, 77, 1103–1124. 10.1007/s00280-016-2976-z. - DOI - PubMed
- Johnstone T. C.; Suntharalingam K.; Lippard S. J. The Next Generation of Platinum Drugs: Targeted Pt(II) Agents, Nanoparticle Delivery, and Pt(IV) Prodrugs. Chem. Rev. 2016, 116, 3436–3486. 10.1021/acs.chemrev.5b00597. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

