Cytotoxicity of Pyrazine-Based Cyclometalated (C^Npz^C)Au(III) Carbene Complexes: Impact of the Nature of the Ancillary Ligand on the Biological Properties
- PMID: 28441013
- PMCID: PMC5434479
- DOI: 10.1021/acs.inorgchem.7b00339
Cytotoxicity of Pyrazine-Based Cyclometalated (C^Npz^C)Au(III) Carbene Complexes: Impact of the Nature of the Ancillary Ligand on the Biological Properties
Abstract
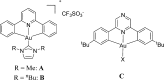
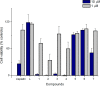
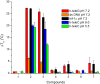
The synthesis of a series of cyclometalated gold(III) complexes supported by pyrazine-based (C^N^C)-type pincer ligands is reported, including the crystal structure of a cationic example. The compounds provide a new platform for the study of antiproliferative properties of gold(III) complexes. Seven complexes were tested: the neutral series (C^Npz^C)AuX [X = Cl (1), 6-thioguanine (4), C≡CPh (5), SPh (6)] and an ionic series that included the N-methyl complex [(C^NpzMe^C)AuCl]BF4 (7) and the N-heterocyclic carbene complexes [(C^Npz^C)AuL]+ with L = 1,3-dimethylbenzimidazol-2-ylidene (2) or 1,3,7,9-tetramethylxanthin-8-ylidene (3). Tests against human leukemia cells identified 1, 2, 3, and 4 as particularly promising, whereas protecting the noncoordinated N atom on the pyrazine ring by methylation (as in 7) reduced the cytotoxicity. Complex 2 proved to be the most effective of the entire series against the HL60 leukemia, MCF-7 breast cancer, and A549 lung cancer cell lines, with IC50 values down to submicromolar levels, associated with a lower toxicity toward healthy human lung fibroblast cells. The benzimidazolylidene complex 2 accumulated more effectively in human lung cancer cells than its caffeine-based analogue 3 and the gold(III) chloride 1. Compound 2 proved to be unaffected by glutathione under physiological conditions for periods of up to 6 days and stabilizes the DNA G-quadruplex and i-motif structures; the latter is the first such report for gold compounds. We also show the first evidence of inhibition of MDM2-p53 protein-protein interactions by a gold-based compound and identified the binding mode of the compound with MDM2 using saturation transfer difference NMR spectroscopy combined with docking calculations.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
-
- Bosl G. J.; Bajorin D. F.; Sheinfeld J.. Cancer of the Testis; DeVita V. T. J., Hellman S., Rosenberg S. A., Eds.; Lippincott Williams & Wilkins: Philadelphia, 2001.
- Watson M.; Barrett A.; Spence R.; Twelves C.. Oncology, 2nd ed.; Oxford University Press: Oxford, U.K., 2006.
-
- Rabik C. A.; Dolan M. E. Molecular mechanisms of resistance and toxicity associated with platinating agents. Cancer Treat. Rev. 2007, 33, 9–23. 10.1016/j.ctrv.2006.09.006. - DOI - PMC - PubMed
- Dilruba S.; Kalayda G. V. Platinum-based drugs: past, present and future. Cancer Chemother. Pharmacol. 2016, 77, 1103–1124. 10.1007/s00280-016-2976-z. - DOI - PubMed
- Johnstone T. C.; Suntharalingam K.; Lippard S. J. The Next Generation of Platinum Drugs: Targeted Pt(II) Agents, Nanoparticle Delivery, and Pt(IV) Prodrugs. Chem. Rev. 2016, 116, 3436–3486. 10.1021/acs.chemrev.5b00597. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

