A population-based approach for implementing change from opt-out to opt-in research permissions
- PMID: 28441388
- PMCID: PMC5404843
- DOI: 10.1371/journal.pone.0168223
A population-based approach for implementing change from opt-out to opt-in research permissions
Abstract
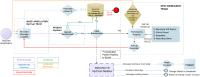
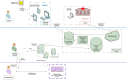
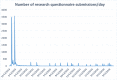
Due to recently proposed changes in the Common Rule regarding the collection of research preferences, there is an increased need for efficient methods to document opt-in research preferences at a population level. Previously, our institution developed an opt-out paper-based workflow that could not be utilized for research in a scalable fashion. This project was designed to demonstrate the feasibility of implementing an electronic health record (EHR)-based active opt-in research preferences program. The first phase of implementation required creating and disseminating a patient questionnaire through the EHR portal to populate discreet fields within the EHR indicating patients' preferences for future research study contact (contact) and their willingness to allow anonymised use of excess tissue and fluid specimens (biobank). In the second phase, the questionnaire was presented within a clinic nurse intake workflow in an obstetrical clinic. These permissions were tabulated in registries for use by investigators for feasibility studies and recruitment. The registry was also used for research patient contact management using a new EHR encounter type to differentiate research from clinical encounters. The research permissions questionnaire was sent to 59,670 patients via the EHR portal. Within four months, 21,814 responses (75% willing to participate in biobanking, and 72% willing to be contacted for future research) were received. Each response was recorded within a patient portal encounter to enable longitudinal analysis of responses. We obtained a significantly lower positive response from the 264 females who completed the questionnaire in the obstetrical clinic (55% volunteers for biobank and 52% for contact). We demonstrate that it is possible to establish a research permissions registry using the EHR portal and clinic-based workflows. This patient-centric, population-based, opt-in approach documents preferences in the EHR, allowing linkage of these preferences to health record information.
Conflict of interest statement
Figures






References
-
- Imagine MUSC 2020 [Internet]. [cited 3 Jun 2016]. http://www.imaginemusc.com/
-
- Overby CL, Maloney KA, Alestock TD, Chavez J, Berman D, Sharaf RM, et al. Prioritizing Approaches to Engage Community Members and Build Trust in Biobanks: A Survey of Attitudes and Opinions of Adults within Outpatient Practices at the University of Maryland. J Pers Med. 2015;5: 264–279. 10.3390/jpm5030264 - DOI - PMC - PubMed
-
- Emanuel EJ. Reform of Clinical Research Regulations, Finally. N Engl J Med. 2015; - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

