Dysbiosis of intestinal microbiota and decrease in paneth cell antimicrobial peptide level during acute necrotizing pancreatitis in rats
- PMID: 28441432
- PMCID: PMC5404871
- DOI: 10.1371/journal.pone.0176583
Dysbiosis of intestinal microbiota and decrease in paneth cell antimicrobial peptide level during acute necrotizing pancreatitis in rats
Abstract
Objectives: Intestinal barrier dysfunction plays an important role in acute necrotizing pancreatitis (ANP) and intestinal microbiota dysbiosis was involved in intestinal barrier failure. Paneth cells protect intestinal barrier and are associated with intestinal microbiota. Here, we investigated changes in intestinal microbiota and antimicrobial peptides of Paneth cells in ileum during ANP.
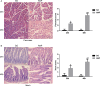
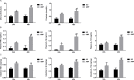
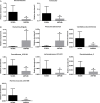
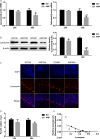
Methods: Rats with ANP were established by retrograde injection of 3.5% sodium taurocholate into biliopancreatic duct and sacrificed at 24h and 48h, respectively. Injuries of pancreas and distal ileum were evaluated by histopathological score. Intestinal barrier function was assessed by plasma diamine oxidase activity (DAO) and D-lactate. Systemic and intestinal inflammation was evaluated by TNFα, IL-1β and IL-17A concentration by ELISA, respectively. 16S rRNA high throughput sequencing on fecal samples was used to investigate the changes in intestinal microbiota in the ANP group at 48h. Lysozyme and α-defensin5 were measured by real-time PCR, western blot and immunofluoresence.
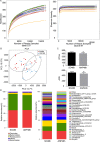
Results: ANP rats had more severe histopathological injuries in pancreas and distal ileum, injured intestinal barrier and increased expression of TNFα, IL-1β and IL-17A in plasma and distal ileum compared with those of the sham-operated (SO) group. Principal component analysis (PCA) showed structural segregation between the SO and ANP groups. Operational taxonomic unit (OTU) number and ACE index revealed decreased microbiota diversity in the ANP group. Taxonomic analysis showed dysbiosis of intestinal microbiota structure. At phyla level, Saccharibacteria and Tenericutes decreased significantly. At genus level, Escherichia-Shigella and Phascolarctobacterium increased significantly, while Candidatus_Saccharimonas, Prevotellaceae_UCG-001, Lachnospiraceae_UCG-001, Ruminiclostridium_5 and Ruminococcaceae_UCG-008 decreased significantly. Lysozyme and α-defensin5 mRNA expression levels decreased significantly in ANP group at 48h. Protein expression of lysozyme decreased in ANP groups at 24h and 48h. Meanwhile, the relative abundance of Escherichia-Shigella correlated inversely with the decrease in lysozyme.
Conclusion: The disorder in intestinal microbiota and decreases of Paneth cell antimicrobial peptides might participate in the pathogenesis of intestinal barrier dysfunction during ANP.
Conflict of interest statement
Figures
References
-
- Qiu Z, Yu P, Bai B, Hao Y, Wang S, Zhao Z, et al. Regulatory B10 cells play a protective role in severe acute pancreatitis. INFLAMM RES. 2016;65(8):647–54. doi: 10.1007/s00011-016-0947-9 - DOI - PubMed
-
- Xu GF, Guo M, Tian ZQ, Wu GZ, Zou XP, Zhang WJ. Increased of serum high-mobility group box chromosomal protein 1 correlated with intestinal mucosal barrier injury in patients with severe acute pancreatitis. WORLD J EMERG SURG. 2014;9:61 doi: 10.1186/1749-7922-9-61 - DOI - PMC - PubMed
-
- Wang YL, Zheng YJ, Zhang ZP, Su JY, Lei RQ, Tang YQ, et al. Effects of gut barrier dysfunction and NF-kappaB activation on aggravating mechanism of severe acute pancreatitis. J Dig Dis. 2009;10(1):30–40. doi: 10.1111/j.1751-2980.2008.00360.x - DOI - PubMed
-
- Zhang XP, Zhang J, Song QL, Chen HQ. Mechanism of acute pancreatitis complicated with injury of intestinal mucosa barrier. J Zhejiang Univ Sci B. 2007;8(12):888–95. doi: 10.1631/jzus.2007.B0888 - DOI - PMC - PubMed
-
- Tan C, Ling Z, Huang Y, Cao Y, Liu Q, Cai T, et al. Dysbiosis of Intestinal Microbiota Associated With Inflammation Involved in the Progression of Acute Pancreatitis. PANCREAS. 2015;44(6):868–75. doi: 10.1097/MPA.0000000000000355 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

