Effectiveness and safety of mycophenolate mofetil in idiopathic pulmonary fibrosis
- PMID: 28441449
- PMCID: PMC5404863
- DOI: 10.1371/journal.pone.0176312
Effectiveness and safety of mycophenolate mofetil in idiopathic pulmonary fibrosis
Abstract
Background: Currently available antifibrotic treatments may slow down disease progression in idiopathic pulmonary fibrosis (IPF), but are associated with potentially significant side effects and are costly. Mycophenolate mofetil (MMF) is well known for its potent immunosuppressive properties and possesses important antiproliferative and antifibrotic effects. The safety and effectiveness of MMF in IPF is unknown.
Methods: We performed a retrospective multicohort analysis of IPF patients treated with MMF compared to those treated with either ineffective/harmful treatments or no treatment. Longitudinal change in forced vital capacity (FVC) between the groups was analyzed using a mixed model with random intercept and slope allowing for repeated measures within subjects. Categorical change in FVC, median overall survival, and adverse events were also assessed.
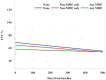
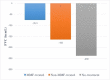
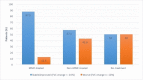
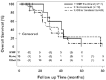
Results: Forty-one IPF patients were included: 11 treated with MMF, 20 treated with ineffective/harmful agents (such as prednisone, azathioprine, and/or NAC), and 10 did not receive any specific treatment for their IPF. After one year, there was a trend towards reduced FVC decline in the MMF-treated group (-76.3 mL, -2.4% of predicted) compared to the non-MMF-treated (-165 mL, -8.9% of predicted) and the no-treatment (-239 mL, -11.5% of predicted) groups, respectively. By categorical change, there was a trend towards greater FVC stability in the MMF-treated group (87.5%) compared to the non-MMF-treated (57%) and the no-treatment groups (50%), respectively. MMF-treated IPF patients had a trend towards improved median overall survival (40.3 months) compared to the non-MMF-treated (25.5 months) and the no-treatment (29.3 months) groups, respectively. Treatment-related adverse events were not different between groups; however, very few adverse events were reported overall.
Conclusions: MMF treatment was associated with potentially clinically important trends toward reduced annual FVC decline (similar to approved antifibrotics), greater FVC stability and improved overall survival in IPF patients. MMF was generally safe, well tolerated, and relatively inexpensive. Future prospective studies of MMF in combination with antifibrotic therapy in IPF are needed.
Conflict of interest statement
Figures
References
-
- Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, et al. An Official ATS/ERS/JRS/ALAT Statement: Idiopathic Pulmonary Fibrosis: Evidence-based Guidelines for Diagnosis and Management. Am J Respir Crit Care Med 2011; 183:788–824. doi: 10.1164/rccm.2009-040GL - DOI - PMC - PubMed
-
- Olson AL, Swigris JJ, Lezotte DC, Norris JM, Wilson CG, Brown KK. Mortality from pulmonary fibrosis increased in the United States from 1992 to 2003. Am J Respir Crit Care Med 2007; 176:277–284. doi: 10.1164/rccm.200701-044OC - DOI - PubMed
-
- American Thoracic Society. Idiopathic pulmonary fibrosis: diagnosis and treatment: international consensus statement. Am J Respir Crit Care Med 2000; 161:646–664. doi: 10.1164/ajrccm.161.2.ats3-00 - DOI - PubMed
-
- Demedts M, Behr J, Buhi R, Costabel U, Dekhuijzen R, Jansen HM, et al. High-dose acetylcysteine in idiopathic pulmonary fibrosis. N Engl J Med 2005; 353:2229–2242. doi: 10.1056/NEJMoa042976 - DOI - PubMed
-
- Raghu G, Anstrom KJ, King TE Jr, Lasky JA, Martinez FJ on behalf of the Idiopathic Pulmonary Fibrosis Clinical Research Network. Prednisone, azathioprine, and N-acetylcysteine for pulmonary fibrosis. N Engl J Med 2012; 366:1968–1977. doi: 10.1056/NEJMoa1113354 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

