Crucial roles of Pox neuro in the developing ellipsoid body and antennal lobes of the Drosophila brain
- PMID: 28441464
- PMCID: PMC5404782
- DOI: 10.1371/journal.pone.0176002
Crucial roles of Pox neuro in the developing ellipsoid body and antennal lobes of the Drosophila brain
Abstract
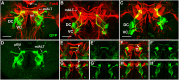
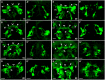
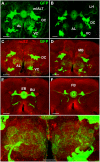
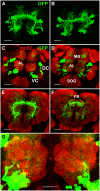
The paired box gene Pox neuro (Poxn) is expressed in two bilaterally symmetric neuronal clusters of the developing adult Drosophila brain, a protocerebral dorsal cluster (DC) and a deutocerebral ventral cluster (VC). We show that all cells that express Poxn in the developing brain are postmitotic neurons. During embryogenesis, the DC and VC consist of only 20 and 12 neurons that express Poxn, designated embryonic Poxn-neurons. The number of Poxn-neurons increases only during the third larval instar, when the DC and VC increase dramatically to about 242 and 109 Poxn-neurons, respectively, virtually all of which survive to the adult stage, while no new Poxn-neurons are added during metamorphosis. Although the vast majority of Poxn-neurons express Poxn only during third instar, about half of them are born by the end of embryogenesis, as demonstrated by the absence of BrdU incorporation during larval stages. At late third instar, embryonic Poxn-neurons, which begin to express Poxn during embryogenesis, can be easily distinguished from embryonic-born and larval-born Poxn-neurons, which begin to express Poxn only during third instar, (i) by the absence of Pros, (ii) their overt differentiation of axons and neurites, and (iii) the strikingly larger diameter of their cell bodies still apparent in the adult brain. The embryonic Poxn-neurons are primary neurons that lay out the pioneering tracts for the secondary Poxn-neurons, which differentiate projections and axons that follow those of the primary neurons during metamorphosis. The DC and the VC participate only in two neuropils of the adult brain. The DC forms most, if not all, of the neurons that connect the bulb (lateral triangle) with the ellipsoid body, a prominent neuropil of the central complex, while the VC forms most of the ventral projection neurons of the antennal lobe, which connect it ipsilaterally to the lateral horn, bypassing the mushroom bodies. In addition, Poxn-neurons of the VC are ventral local interneurons of the antennal lobe. In the absence of Poxn protein in the developing brain, embryonic Poxn-neurons stall their projections and cannot find their proper target neuropils, the bulb and ellipsoid body in the case of the DC, or the antennal lobe and lateral horn in the case of the VC, whereby the absence of the ellipsoid body neuropil is particularly striking. Poxn is thus crucial for pathfinding both in the DC and VC. Additional implications of our results are discussed.
Conflict of interest statement
Figures






Similar articles
-
Multiple function of poxn gene in larval PNS development and in adult appendage formation of Drosophila.Dev Genes Evol. 2001 Jan;211(1):20-9. doi: 10.1007/s004270000119. Dev Genes Evol. 2001. PMID: 11277402
-
The paired box gene pox neuro: a determinant of poly-innervated sense organs in Drosophila.Cell. 1992 Apr 3;69(1):159-72. doi: 10.1016/0092-8674(92)90127-x. Cell. 1992. PMID: 1348214
-
Developmental regulation and functions of the expression of the neuropeptide corazonin in Drosophila melanogaster.Cell Tissue Res. 2008 Mar;331(3):659-73. doi: 10.1007/s00441-007-0549-5. Epub 2007 Dec 18. Cell Tissue Res. 2008. PMID: 18087727
-
Using Pox-Neuro (Poxn) Mutants in Drosophila Gustation Research: A Double-Edged Sword.Front Cell Neurosci. 2018 Oct 24;12:382. doi: 10.3389/fncel.2018.00382. eCollection 2018. Front Cell Neurosci. 2018. PMID: 30405359 Free PMC article. Review.
-
Growth and Maturation in Development: A Fly's Perspective.Int J Mol Sci. 2020 Feb 13;21(4):1260. doi: 10.3390/ijms21041260. Int J Mol Sci. 2020. PMID: 32070061 Free PMC article. Review.
Cited by
-
Sensory mutations in Drosophila melanogaster influence associational effects between resources during oviposition.Sci Rep. 2017 Aug 24;7(1):9352. doi: 10.1038/s41598-017-09728-7. Sci Rep. 2017. PMID: 28839208 Free PMC article.
-
Neuronal Constituents and Putative Interactions Within the Drosophila Ellipsoid Body Neuropil.Front Neural Circuits. 2018 Nov 27;12:103. doi: 10.3389/fncir.2018.00103. eCollection 2018. Front Neural Circuits. 2018. PMID: 30546298 Free PMC article.
-
Hierarchical diversification of instinctual behavior neurons by lineage, birth order, and sex.bioRxiv [Preprint]. 2025 Jun 3:2025.06.03.657692. doi: 10.1101/2025.06.03.657692. bioRxiv. 2025. PMID: 40502082 Free PMC article. Preprint.
-
Decoding gene regulation in the fly brain.Nature. 2022 Jan;601(7894):630-636. doi: 10.1038/s41586-021-04262-z. Epub 2022 Jan 5. Nature. 2022. PMID: 34987221
-
A Single-Cell Transcriptome Atlas of the Aging Drosophila Brain.Cell. 2018 Aug 9;174(4):982-998.e20. doi: 10.1016/j.cell.2018.05.057. Epub 2018 Jun 18. Cell. 2018. PMID: 29909982 Free PMC article.
References
-
- Ito K, Hotta Y (1992) Proliferation pattern of postembryonic neuroblasts in the brain of Drosophila melanogaster. Dev Biol 149: 134–148. - PubMed
-
- Levine RB, Morton DB, Restifo LL (1995) Remodeling of the insect nervous system. Curr Opin Neurobiol 5: 28–35. - PubMed
-
- Consoulas C, Duch C, Bayline RJ, Levine RB (2000) Behavioral transformations during metamorphosis: Remodeling of neural and motor systems. Brain Res Bull 53: 571–583. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

