Multiple UDP-Glucuronosyltransferase and Sulfotransferase Enzymes are Responsible for the Metabolism of Verproside in Human Liver Preparations
- PMID: 28441724
- PMCID: PMC6154560
- DOI: 10.3390/molecules22040670
Multiple UDP-Glucuronosyltransferase and Sulfotransferase Enzymes are Responsible for the Metabolism of Verproside in Human Liver Preparations
Abstract
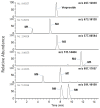
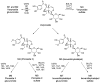
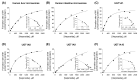
Verproside, an active iridoid glycoside component of Veronica species, such as Pseudolysimachion rotundum var. subintegrum and Veronica anagallis-aquatica, possesses anti-asthma, anti-inflammatory, anti-nociceptive, antioxidant, and cytostatic activities. Verproside is metabolized into nine metabolites in human hepatocytes: verproside glucuronides (M1, M2) via glucuronidation, verproside sulfate (M3) via sulfation, picroside II (M4) and isovanilloylcatalpol (M5) via O-methylation, M4 glucuronide (M6) and M4 sulfate (M8) via further glucuronidation and sulfation of M4, and M5 glucuronide (M7) and M5 sulfate (M9) via further glucuronidation and sulfation of M5. Drug-metabolizing enzymes responsible for verproside metabolism, including sulfotransferase (SULT) and UDP-glucuronosyltransferase (UGT), were characterized. The formation of verproside glucuronides (M1, M2), isovanilloylcatalpol glucuronide (M7), and picroside II glucuronide (M6) was catalyzed by commonly expressed UGT1A1 and UGT1A9 and gastrointestinal-specific UGT1A7, UGT1A8, and UGT1A10, consistent with the higher intrinsic clearance values for the formation of M1, M2, M6, and M7 in human intestinal microsomes compared with those in liver microsomes. The formation of verproside sulfate (M3) and M5 sulfate (M9) from verproside and isovanilloylcatalpol (M5), respectively, was catalyzed by SULT1A1. Metabolism of picroside II (M4) into M4 sulfate (M8) was catalyzed by SULT1A1, SULT1E1, SULT1A2, SULT1A3, and SULT1C4. Based on these results, the pharmacokinetics of verproside may be affected by the co-administration of relevant UGT and SULT inhibitors or inducers.
Keywords: UDP-glucuronosyltransferase; in vitro metabolism; sulfotransferase; verproside.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



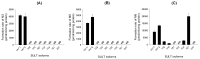



Similar articles
-
Identification of Catalposide Metabolites in Human Liver and Intestinal Preparations and Characterization of the Relevant Sulfotransferase, UDP-glucuronosyltransferase, and Carboxylesterase Enzymes.Pharmaceutics. 2019 Jul 22;11(7):355. doi: 10.3390/pharmaceutics11070355. Pharmaceutics. 2019. PMID: 31336576 Free PMC article.
-
In vitro and in vivo metabolism of verproside in rats.Molecules. 2012 Oct 12;17(10):11990-2002. doi: 10.3390/molecules171011990. Molecules. 2012. PMID: 23085650 Free PMC article.
-
Assessment of UDP-glucuronosyltransferase catalyzed formation of Picroside II glucuronide in microsomes of different species and recombinant UGTs.Xenobiotica. 2011 Jul;41(7):530-7. doi: 10.3109/00498254.2011.573018. Epub 2011 Apr 27. Xenobiotica. 2011. PMID: 21524190
-
Regioselective sulfation and glucuronidation of phenolics: insights into the structural basis.Curr Drug Metab. 2011 Nov;12(9):900-16. doi: 10.2174/138920011797470100. Curr Drug Metab. 2011. PMID: 21933112 Free PMC article. Review.
-
The Role of Uptake and Efflux Transporters in the Disposition of Glucuronide and Sulfate Conjugates.Front Pharmacol. 2022 Jan 13;12:802539. doi: 10.3389/fphar.2021.802539. eCollection 2021. Front Pharmacol. 2022. PMID: 35095509 Free PMC article. Review.
Cited by
-
Identification of Catalposide Metabolites in Human Liver and Intestinal Preparations and Characterization of the Relevant Sulfotransferase, UDP-glucuronosyltransferase, and Carboxylesterase Enzymes.Pharmaceutics. 2019 Jul 22;11(7):355. doi: 10.3390/pharmaceutics11070355. Pharmaceutics. 2019. PMID: 31336576 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

