Polyphenolic Compounds and Digestive Enzymes: In Vitro Non-Covalent Interactions
- PMID: 28441731
- PMCID: PMC6154557
- DOI: 10.3390/molecules22040669
Polyphenolic Compounds and Digestive Enzymes: In Vitro Non-Covalent Interactions
Abstract
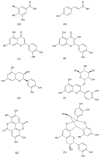
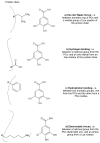
The digestive enzymes-polyphenolic compounds (PCs) interactions behind the inhibition of these enzymes have not been completely studied. The existing studies have mainly analyzed polyphenolic extracts and reported inhibition percentages of catalytic activities determined by UV-Vis spectroscopy techniques. Recently, pure PCs and new methods such as isothermal titration calorimetry and circular dichroism have been applied to describe these interactions. The present review focuses on PCs structural characteristics behind the inhibition of digestive enzymes, and progress of the used methods. Some characteristics such as molecular weight, number and position of substitution, and glycosylation of flavonoids seem to be related to the inhibitory effect of PCs; also, this effect seems to be different for carbohydrate-hydrolyzing enzymes and proteases. The digestive enzyme-PCs molecular interactions have shown that non-covalent binding, mostly by van der Waals forces, hydrogen binding, hydrophobic binding, and other electrostatic forces regulate them. These interactions were mainly associated to non-competitive type inhibitions of the enzymatic activities. The present review emphasizes on the digestive enzymes such as α-glycosidase (AG), α-amylase (PA), lipase (PL), pepsin (PE), trypsin (TP), and chymotrypsin (CT). Existing studies conducted in vitro allow one to elucidate the characteristics of the structure-function relationships, where differences between the structures of PCs might be the reason for different in vivo effects.
Keywords: digestive enzymes; enzymatic inhibition; hydrogen binding; hydrophobic binding; polyphenolic compounds; structure; van der Waals forces.
Conflict of interest statement
The authors declare that no conflicts of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

