Modified Nucleotides as Substrates of Terminal Deoxynucleotidyl Transferase
- PMID: 28441732
- PMCID: PMC6154577
- DOI: 10.3390/molecules22040672
Modified Nucleotides as Substrates of Terminal Deoxynucleotidyl Transferase
Abstract
The synthesis of novel modified nucleotides and their incorporation into DNA sequences opens many possibilities to change the chemical properties of oligonucleotides (ONs), and, therefore, broaden the field of practical applications of modified DNA. The chemical synthesis of nucleotide derivatives, including ones bearing thio-, hydrazino-, cyano- and carboxy groups as well as 2-pyridone nucleobase-containing nucleotides was carried out. The prepared compounds were tested as substrates of terminal deoxynucleotidyl transferase (TdT). The nucleotides containing N⁴-aminocytosine, 4-thiouracil as well as 2-pyridone, 4-chloro- and 4-bromo-2-pyridone as a nucleobase were accepted by TdT, thus allowing enzymatic synthesis of 3'-terminally modified ONs. The successful UV-induced cross-linking of 4-thiouracil-containing ONs to TdT was carried out. Enzymatic post-synthetic 3'-modification of ONs with various photo- and chemically-reactive groups opens novel possibilities for future applications, especially in analysis of the mechanisms of polymerases and the development of photo-labels, sensors, and self-assembling structures.
Keywords: 4-bromo-2-pyridone; 4-chloro-2-pyridone; 4-thio-2’-deoxyuridine triphosphate; N4-amino-2’-deoxycytidine triphosphate; UV cross-linking; terminal deoxynucleotidyl transferase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

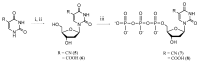

References
-
- Kraemer S., Vaught J.D., Bock C., Gold L., Katilius E., Keeney T.R., Kim N., Saccomano N.A., Wilcox S.K., Zichi D., et al. From SOMAmer-based biomarker discovery to diagnostic and clinical applications: A SOMAmer-based, streamlined multiplex proteomic assay. PLoS ONE. 2011;6:e26332. doi: 10.1371/journal.pone.0026332. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

