CoQ10 Deficiency May Indicate Mitochondrial Dysfunction in Cr(VI) Toxicity
- PMID: 28441753
- PMCID: PMC5412400
- DOI: 10.3390/ijms18040816
CoQ10 Deficiency May Indicate Mitochondrial Dysfunction in Cr(VI) Toxicity
Abstract
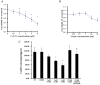
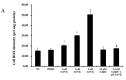
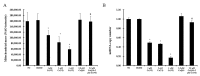
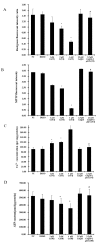
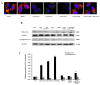
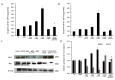
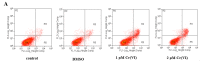
To investigate the toxic mechanism of hexavalent chromium Cr(VI) and search for an antidote for Cr(VI)-induced cytotoxicity, a study of mitochondrial dysfunction induced by Cr(VI) and cell survival by recovering mitochondrial function was performed. In the present study, we found that the gene expression of electron transfer flavoprotein dehydrogenase (ETFDH) was strongly downregulated by Cr(VI) exposure. The levels of coenzyme 10 (CoQ10) and mitochondrial biogenesis presented by mitochondrial mass and mitochondrial DNA copy number were also significantly reduced after Cr(VI) exposure. The subsequent, Cr(VI)-induced mitochondrial damage and apoptosis were characterized by reactive oxygen species (ROS) accumulation, caspase-3 and caspase-9 activation, decreased superoxide dismutase (SOD) and ATP production, increased methane dicarboxylic aldehyde (MDA) content, mitochondrial membrane depolarization and mitochondrial permeability transition pore (MPTP) opening, increased Ca2+ levels, Cyt c release, decreased Bcl-2 expression, and significantly elevated Bax expression. The Cr(VI)-induced deleterious changes were attenuated by pretreatment with CoQ10 in L-02 hepatocytes. These data suggest that Cr(VI) induces CoQ10 deficiency in L-02 hepatocytes, indicating that this deficiency may be a biomarker of mitochondrial dysfunction in Cr(VI) poisoning and that exogenous administration of CoQ10 may restore mitochondrial function and protect the liver from Cr(VI) exposure.
Keywords: L-02 hepatocytes; apoptosis; coenzyme Q10; hexavalent chromium Cr(VI); mitochondrial membrane potential (MMP); reactive oxygen species (ROS).
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Hexavalent chromium induces oxidative stress and mitochondria-mediated apoptosis in isolated skin fibroblasts of Indo-Pacific humpback dolphin.Aquat Toxicol. 2018 Oct;203:179-186. doi: 10.1016/j.aquatox.2018.08.012. Epub 2018 Aug 20. Aquat Toxicol. 2018. PMID: 30153559
-
Hexavalent chromium targets mitochondrial respiratory chain complex I to induce reactive oxygen species-dependent caspase-3 activation in L-02 hepatocytes.Int J Mol Med. 2012 Sep;30(3):629-35. doi: 10.3892/ijmm.2012.1031. Epub 2012 Jun 14. Int J Mol Med. 2012. PMID: 22710416
-
Cr(VI) induces cytotoxicity in vitro through activation of ROS-mediated endoplasmic reticulum stress and mitochondrial dysfunction via the PI3K/Akt signaling pathway.Toxicol In Vitro. 2017 Jun;41:232-244. doi: 10.1016/j.tiv.2017.03.003. Epub 2017 Mar 18. Toxicol In Vitro. 2017. PMID: 28323103
-
Effects of hexavalent chromium on mitochondria and their implications in carcinogenesis.J Environ Sci Health C Toxicol Carcinog. 2024;42(2):109-125. doi: 10.1080/26896583.2024.2301899. Epub 2024 Jan 17. J Environ Sci Health C Toxicol Carcinog. 2024. PMID: 38230947 Review.
-
Toxic and carcinogenic effects of hexavalent chromium in mammalian cells in vivo and in vitro: a recent update.J Environ Sci Health C Toxicol Carcinog. 2022;40(3-4):282-315. doi: 10.1080/26896583.2022.2158675. Epub 2023 Feb 2. J Environ Sci Health C Toxicol Carcinog. 2022. PMID: 36728911 Review.
Cited by
-
Research progress of mitochondrial dysfunction induced pyroptosis in acute lung injury.Respir Res. 2024 Nov 7;25(1):398. doi: 10.1186/s12931-024-03028-1. Respir Res. 2024. PMID: 39511593 Free PMC article. Review.
-
The Scavenging Activity of Coenzyme Q10 Plus a Nutritional Complex on Human Retinal Pigment Epithelial Cells.Int J Mol Sci. 2024 Jul 24;25(15):8070. doi: 10.3390/ijms25158070. Int J Mol Sci. 2024. PMID: 39125641 Free PMC article.
-
Antioxidant Effect of Coenzyme Q10 in the Prevention of Oxidative Stress in Arsenic-Treated CHO-K1 Cells and Possible Participation of Zinc as a Pro-Oxidant Agent.Nutrients. 2022 Aug 10;14(16):3265. doi: 10.3390/nu14163265. Nutrients. 2022. PMID: 36014770 Free PMC article.
-
Coenzyme Q10 Attenuates Kidney Injury Induced by Titanium Dioxide Nanoparticles and Cadmium Co-exposure in Rats.Biol Trace Elem Res. 2025 Aug;203(8):4183-4197. doi: 10.1007/s12011-024-04469-x. Epub 2024 Dec 21. Biol Trace Elem Res. 2025. PMID: 39707081 Free PMC article.
-
Adaptive responses to neurodegenerative stress in glaucoma.Prog Retin Eye Res. 2021 Sep;84:100953. doi: 10.1016/j.preteyeres.2021.100953. Epub 2021 Feb 25. Prog Retin Eye Res. 2021. PMID: 33640464 Free PMC article. Review.
References
-
- Stout M.D., Herbert R.A., Kissling G.E., Collins B.J., Travlos G.S., Witt K.L., Melnick R.L., Abdo K.M., Malarkey D.E., Hooth M.J. Hexavalent chromium is carcinogenic to F344/N rats and B6C3F1 mice after chronic oral exposure. Environ. Health Perspect. 2009;117:716–722. doi: 10.1289/ehp.0800208. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

