Role of RHEB in Regulating Differentiation Fate of Mesenchymal Stem Cells for Cartilage and Bone Regeneration
- PMID: 28441755
- PMCID: PMC5412461
- DOI: 10.3390/ijms18040880
Role of RHEB in Regulating Differentiation Fate of Mesenchymal Stem Cells for Cartilage and Bone Regeneration
Abstract
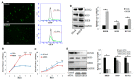
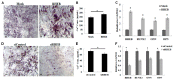
Advances in mesenchymal stem cells (MSCs) and cell replacement therapies are promising approaches to treat cartilage and bone defects since substantial differentiation capacities of MSCs match the demands of tissue regeneration. Our understanding of the dynamic process requiring indispensable differentiation of MSCs remains limited. Herein, we describe the role of RHEB (Ras homolog enriched in brain) regulating gene signature for differentiation of human adipose derived mesenchymal stem cells (ASCs) into chondrogenic, osteogenic, and adipogenic lineages. RHEB-overexpression increases the proliferation of the ASCs. RHEB enhances the chondrogenic differentiation of ASCs in 3D culture via upregulation of SOX9 with concomitant increase in glycosaminoglycans (GAGs), and type II collagen (COL2). RHEB increases the osteogenesis via upregulation of runt related transcription factor 2 (RUNX2) with an increase in the calcium and phosphate contents. RHEB also increases the expression of osteogenic markers, osteonectin and osteopontin. RHEB knockdown ASCs were incapable of expressing sufficient SRY (Sex determining region Y)-box 9 (SOX9) and RUNX2, and therefore had decreased chondrogenic and osteogenic differentiation. RHEB-overexpression impaired ASCs differentiation into adipogenic lineage, through downregulation of CCAAT/enhancer binding protein beta (C/EBPβ). Conversely, RHEB knockdown abolished the negative regulation of adipogenesis. We demonstrate that RHEB is a novel regulator, with a critical role in ASCs lineage determination, and RHEB-modulated ASCs may be useful as a cell therapy for cartilage and bone defect treatments.
Keywords: Ras homolog enriched in brain (RHEB); adipogenesis; chondrogenesis; differentiation; mesenchymal stem cells; osteogenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Tissue source determines the differentiation potentials of mesenchymal stem cells: a comparative study of human mesenchymal stem cells from bone marrow and adipose tissue.Stem Cell Res Ther. 2017 Dec 6;8(1):275. doi: 10.1186/s13287-017-0716-x. Stem Cell Res Ther. 2017. PMID: 29208029 Free PMC article.
-
Donor-matched mesenchymal stem cells from knee infrapatellar and subcutaneous adipose tissue of osteoarthritic donors display differential chondrogenic and osteogenic commitment.Eur Cell Mater. 2014 Apr 23;27:298-311. doi: 10.22203/ecm.v027a21. Eur Cell Mater. 2014. PMID: 24760577
-
SOX9 gene transfer via safe, stable, replication-defective recombinant adeno-associated virus vectors as a novel, powerful tool to enhance the chondrogenic potential of human mesenchymal stem cells.Stem Cell Res Ther. 2012;3(3):22. doi: 10.1186/scrt113. Stem Cell Res Ther. 2012. PMID: 22742415 Free PMC article.
-
[The Role of Histone Demethylase in Osteogenic and Chondrogenic Differentiation of Mesenchymal Stem Cells: A Literature Review].Sichuan Da Xue Xue Bao Yi Xue Ban. 2021 May;52(3):364-372. doi: 10.12182/20210560202. Sichuan Da Xue Xue Bao Yi Xue Ban. 2021. PMID: 34018352 Free PMC article. Review. Chinese.
-
Determinants of stem cell lineage differentiation toward chondrogenesis versus adipogenesis.Cell Mol Life Sci. 2019 May;76(9):1653-1680. doi: 10.1007/s00018-019-03017-4. Epub 2019 Jan 28. Cell Mol Life Sci. 2019. PMID: 30689010 Free PMC article. Review.
Cited by
-
RSP5 Positively Regulates the Osteogenic Differentiation of Mesenchymal Stem Cells by Activating the K63-Linked Ubiquitination of Akt.Stem Cells Int. 2020 Apr 6;2020:7073805. doi: 10.1155/2020/7073805. eCollection 2020. Stem Cells Int. 2020. PMID: 32322280 Free PMC article.
-
Knockdown of FOXA2 enhances the osteogenic differentiation of bone marrow-derived mesenchymal stem cells partly via activation of the ERK signalling pathway.Cell Death Dis. 2018 Aug 6;9(8):836. doi: 10.1038/s41419-018-0857-6. Cell Death Dis. 2018. PMID: 30082727 Free PMC article.
-
Dental Pulp Stem Cells for Bone Tissue Engineering: A Literature Review.Stem Cells Int. 2023 Oct 11;2023:7357179. doi: 10.1155/2023/7357179. eCollection 2023. Stem Cells Int. 2023. PMID: 37868704 Free PMC article. Review.
-
Autologous lipotransfer for bone defects secondary to osteomyelitis: A report of a novel method and systematic review of the literature.Int Wound J. 2019 Aug;16(4):916-924. doi: 10.1111/iwj.13119. Epub 2019 Mar 27. Int Wound J. 2019. PMID: 30916475 Free PMC article.
-
Recent Advance in Source, Property, Differentiation, and Applications of Infrapatellar Fat Pad Adipose-Derived Stem Cells.Stem Cells Int. 2020 Mar 7;2020:2560174. doi: 10.1155/2020/2560174. eCollection 2020. Stem Cells Int. 2020. PMID: 32215015 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

