Natural [4 + 2]-Cyclases
- PMID: 28441874
- PMCID: PMC6063532
- DOI: 10.1021/acs.chemrev.6b00578
Natural [4 + 2]-Cyclases
Abstract
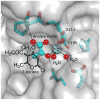
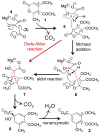
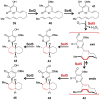
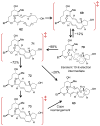
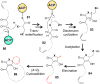
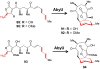
[4 + 2]-Cycloadditions are increasingly being recognized in the biosynthetic pathways of many structurally complex natural products. A relatively small collection of enzymes from these pathways have been demonstrated to increase rates of cyclization and impose stereochemical constraints on the reactions. While mechanistic investigation of these enzymes is just beginning, recent studies have provided new insights with implications for understanding their biosynthetic roles, mechanisms of catalysis, and evolutionary origin.
Figures






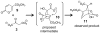




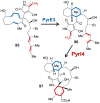
References
-
- Reetz MT. Artificial Metalloenzymes as Catalysts in Stereoselective Diels-Alder Reactions. Chemical Record. 2012;12:391–406. - PubMed
-
- Kries H, Blomberg R, Hilvert D. De Novo Enzymes by Computational Design. Curr Opin Chem Biol. 2013;17:221–228. - PubMed
-
- Mak WS, Siegel JB. Computational Enzyme Design: Transitioning from Catalytic Proteins to Enzymes. Curr Opin Struct Biol. 2014;27:87–94. - PubMed
-
- Tantillo DJ, Chen J, Houk KN. Theozymes and Compuzymes: Theoretical Models for Biological Catalysts. Curr Opin Chem Biol. 1998;2:743–750. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

