Comparison of CSF markers and semi-quantitative amyloid PET in Alzheimer's disease diagnosis and in cognitive impairment prognosis using the ADNI-2 database
- PMID: 28441967
- PMCID: PMC5405503
- DOI: 10.1186/s13195-017-0260-z
Comparison of CSF markers and semi-quantitative amyloid PET in Alzheimer's disease diagnosis and in cognitive impairment prognosis using the ADNI-2 database
Abstract
Background: The relative performance of semi-quantitative amyloid positron emission tomography (PET) and cerebrospinal fluid (CSF) markers in diagnosing Alzheimer's disease (AD) and predicting the cognitive evolution of patients with mild cognitive impairment (MCI) is still debated.
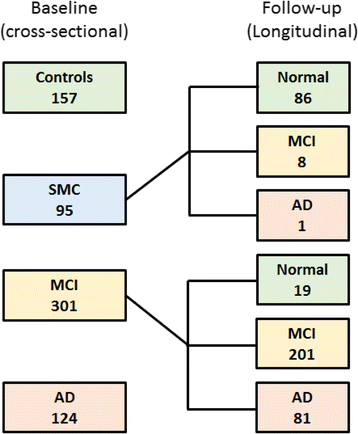
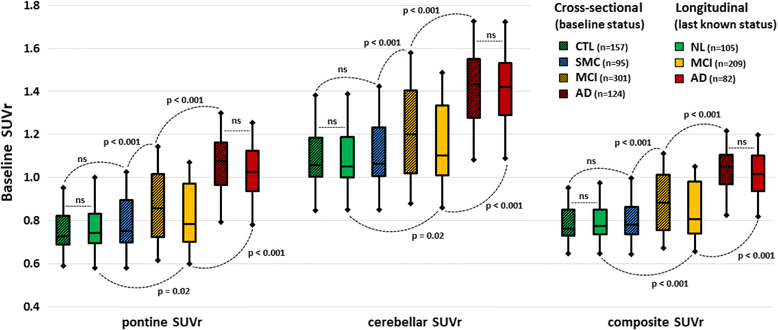
Methods: Subjects from the Alzheimer's Disease Neuroimaging Initiative 2 with complete baseline cognitive assessment (Mini Mental State Examination, Clinical Dementia Rating [CDR] and Alzheimer's Disease Assessment Scale-Cognitive Subscale [ADAS-cog] scores), CSF collection (amyloid-β1-42 [Aβ], tau and phosphorylated tau) and 18F-florbetapir scans were included in our cross-sectional cohort. Among these, patients with MCI or substantial memory complaints constituted our longitudinal cohort and were followed for 30 ± 16 months. PET amyloid deposition was quantified using relative retention indices (standardised uptake value ratio [SUVr]) with respect to pontine, cerebellar and composite reference regions. Diagnostic and prognostic performance based on PET and CSF was evaluated using ROC analysis, multivariate linear regression and survival analysis with the Cox proportional hazards model.
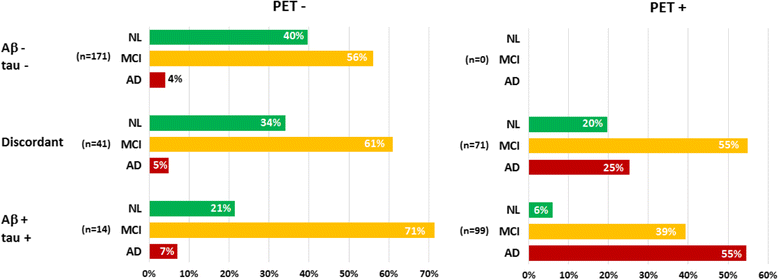
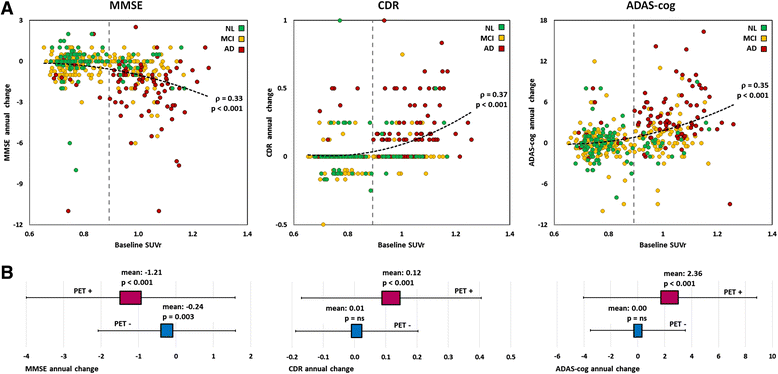
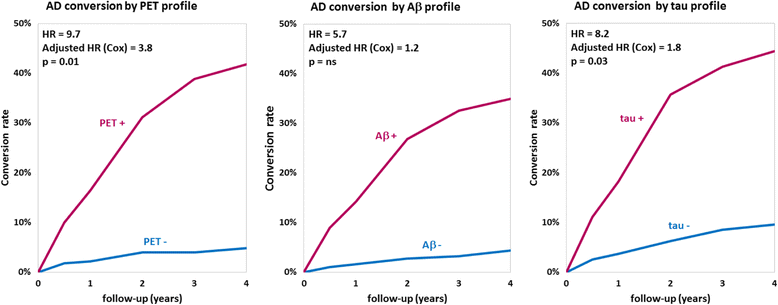
Results: The cross-sectional study included 677 participants and revealed that pontine and composite SUVr values were better classifiers (AUC 0.88, diagnostic accuracy 85%) than CSF markers (AUC 0.83 and 0.85, accuracy 80% and 75%, for Aβ and tau, respectively). SUVr was a strong independent determinant of cognition in multivariate regression, whereas Aβ was not; tau was also a determinant, but to a lesser degree. Among the 396 patients from the longitudinal study, 82 (21%) converted to AD within 22 ± 13 months. Optimal SUVr thresholds to differentiate AD converters were quite similar to those of the cross-sectional study. Composite SUVr was the best AD classifier (AUC 0.86, sensitivity 88%, specificity 81%). In multivariate regression, baseline cognition (CDR and ADAS-cog) was the main predictor of subsequent cognitive decline. Pontine and composite SUVr were moderate but independent predictors of final status and CDR/ADAS-cog progression rate, whereas baseline CSF markers had a marginal influence. The adjusted HRs for AD conversion were 3.8 (p = 0.01) for PET profile, 1.2 (p = ns) for Aβ profile and 1.8 (p = 0.03) for tau profile.
Conclusions: Semi-quantitative amyloid PET appears more powerful than CSF markers for AD grading and MCI prognosis in terms of cognitive decline and AD conversion.
Keywords: ADNI; Alzheimer’s disease; Amyloid PET; CSF markers; MCI.
Figures
References
-
- Ellendt S, Voß B, Kohn N, Wagels L, Goerlich K, Drexler E, et al. Predicting stability of mild cognitive impairment (MCI): findings of a community based sample. Curr Alzheimer Res. doi:10.2174/1567205014666161213120807. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

