Pathogenic CD4+ T cells in patients with asthma
- PMID: 28442213
- PMCID: PMC5651193
- DOI: 10.1016/j.jaci.2017.02.025
Pathogenic CD4+ T cells in patients with asthma
Abstract
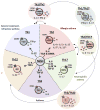
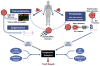
Asthma encompasses a variety of clinical phenotypes that involve distinct T cell-driven inflammatory processes. Improved understanding of human T-cell biology and the influence of innate cytokines on T-cell responses at the epithelial barrier has led to new asthma paradigms. This review captures recent knowledge on pathogenic CD4+ T cells in asthmatic patients by drawing on observations in mouse models and human disease. In patients with allergic asthma, TH2 cells promote IgE-mediated sensitization, airway hyperreactivity, and eosinophilia. Here we discuss recent discoveries in the myriad molecular pathways that govern the induction of TH2 differentiation and the critical role of GATA-3 in this process. We elaborate on how cross-talk between epithelial cells, dendritic cells, and innate lymphoid cells translates to T-cell outcomes, with an emphasis on the actions of thymic stromal lymphopoietin, IL-25, and IL-33 at the epithelial barrier. New concepts on how T-cell skewing and epitope specificity are shaped by multiple environmental cues integrated by dendritic cell "hubs" are discussed. We also describe advances in understanding the origins of atypical TH2 cells in asthmatic patients, the role of TH1 cells and other non-TH2 types in asthmatic patients, and the features of T-cell pathogenicity at the single-cell level. Progress in technologies that enable highly multiplexed profiling of markers within a single cell promise to overcome barriers to T-cell discovery in human asthmatic patients that could transform our understanding of disease. These developments, along with novel T cell-based therapies, position us to expand the assortment of molecular targets that could facilitate personalized treatments.
Keywords: Asthma; GATA-3; IL-25; IL-33; IgE; T(H)1; T(H)17; T(H)2; T(H)22; T-bet; T-cell epitopes; T-cell plasticity; allergens; epithelial barrier; follicular helper T cell; thymic stromal lymphopoietin.
Copyright © 2017 American Academy of Allergy, Asthma & Immunology. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Disclosure of potential conflict of interest: L. M. Muehling received a grant from the National Institutes of Health (NIH) for this and other works and received a student travel award to attend the annual ISAC conference (CYTO 2016) from the International Society for the Advancement of Cytometry. J. A. Woodfolk received a grant from the NIH/National Institute of Allergy and Infectious Diseases and the NIH/National Institute of Arthritis and Musculoskeletal and Skin Diseases for this and other works. M. G. Lawrence declares that she has no relevant conflicts of interest.
Figures


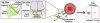

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

