NLRP3 signaling drives macrophage-induced adaptive immune suppression in pancreatic carcinoma
- PMID: 28442553
- PMCID: PMC5461004
- DOI: 10.1084/jem.20161707
NLRP3 signaling drives macrophage-induced adaptive immune suppression in pancreatic carcinoma
Abstract
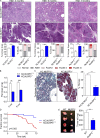
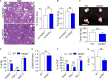
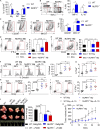
The tumor microenvironment (TME) in pancreatic ductal adenocarcinoma (PDA) is characterized by immune tolerance, which enables disease to progress unabated by adaptive immunity. However, the drivers of this tolerogenic program are incompletely defined. In this study, we found that NLRP3 promotes expansion of immune-suppressive macrophages in PDA. NLRP3 signaling in macrophages drives the differentiation of CD4+ T cells into tumor-promoting T helper type 2 cell (Th2 cell), Th17 cell, and regulatory T cell populations while suppressing Th1 cell polarization and cytotoxic CD8+ T cell activation. The suppressive effects of NLRP3 signaling were IL-10 dependent. Pharmacological inhibition or deletion of NLRP3, ASC (apoptosis-associated speck-like protein containing a CARD complex), or caspase-1 protected against PDA and was associated with immunogenic reprogramming of innate and adaptive immunity within the TME. Similarly, transfer of PDA-entrained macrophages or T cells from NLRP3-/- hosts was protective. These data suggest that targeting NLRP3 holds the promise for the immunotherapy of PDA.
© 2017 Daley et al.
Figures




References
-
- Allen I.C., TeKippe E.M., Woodford R.M., Uronis J.M., Holl E.K., Rogers A.B., Herfarth H.H., Jobin C., and Ting J.P.. 2010. The NLRP3 inflammasome functions as a negative regulator of tumorigenesis during colitis-associated cancer. J. Exp. Med. 207:1045–1056. 10.1084/jem.20100050 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

