β2-Adrenergic receptor activation mobilizes intracellular calcium via a non-canonical cAMP-independent signaling pathway
- PMID: 28442571
- PMCID: PMC5473248
- DOI: 10.1074/jbc.M117.787119
β2-Adrenergic receptor activation mobilizes intracellular calcium via a non-canonical cAMP-independent signaling pathway
Abstract
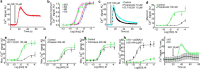
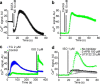
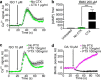
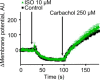
Beta adrenergic receptors (βARs) are G-protein-coupled receptors essential for physiological responses to the hormones/neurotransmitters epinephrine and norepinephrine which are found in the nervous system and throughout the body. They are the targets of numerous widely used drugs, especially in the case of the most extensively studied βAR, β2AR, whose ligands are used for asthma and cardiovascular disease. βARs signal through Gαs G-proteins and via activation of adenylyl cyclase and cAMP-dependent protein kinase, but some alternative downstream pathways have also been proposed that could be important for understanding normal physiological functioning of βAR signaling and its disruption in disease. Using fluorescence-based Ca2+ flux assays combined with pharmacology and gene knock-out methods, we discovered a previously unrecognized endogenous pathway in HEK-293 cells whereby β2AR activation leads to robust Ca2+ mobilization from intracellular stores via activation of phospholipase C and opening of inositol trisphosphate (InsP3) receptors. This pathway did not involve cAMP, Gαs, or Gαi or the participation of the other members of the canonical β2AR signaling cascade and, therefore, constitutes a novel signaling mechanism for this receptor. This newly uncovered mechanism for Ca2+ mobilization by β2AR has broad implications for adrenergic signaling, cross-talk with other signaling pathways, and the effects of βAR-directed drugs.
Keywords: G-protein-coupled receptor (GPCR); adrenergic receptor; calcium intracellular release; cell signaling; cyclic AMP (cAMP); β2-adrenergic receptor.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Figures


References
-
- Milligan G., Svoboda P., and Brown C. M. (1994) Why are there so many adrenoceptor subtypes? Biochem. Pharmacol. 48, 1059–1071 - PubMed
-
- Hoffmann C., Leitz M. R., Oberdorf-Maass S., Lohse M. J., and Klotz K. N. (2004) Comparative pharmacology of human β-adrenergic receptor subtypes: characterization of stably transfected receptors in CHO cells. Naunyn Schmiedebergs Arch. Pharmacol. 369, 151–159 - PubMed
-
- Weitl N., and Seifert R. (2008) Distinct interactions of human β1- and β2-adrenoceptors with isoproterenol, epinephrine, norepinephrine, and dopamine. J. Pharmacol. Exp. Ther. 327, 760–769 - PubMed
-
- Bylund D. B., Eikenberg D. C., Hieble J. P., Langer S. Z., Lefkowitz R. J., Minneman K. P., Molinoff P. B., Ruffolo R. R. Jr., and Trendelenburg U. (1994) International Union of Pharmacology nomenclature of adrenoceptors. Pharmacol. Rev. 46, 121–136 - PubMed
-
- Summers R. J., Kompa A., and Roberts S. J. (1997) β-adrenoceptor subtypes and their desensitization mechanisms. J. Auton. Pharmacol. 17, 331–343 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

