The phospholipase iPLA2γ is a major mediator releasing oxidized aliphatic chains from cardiolipin, integrating mitochondrial bioenergetics and signaling
- PMID: 28442572
- PMCID: PMC5481572
- DOI: 10.1074/jbc.M117.783068
The phospholipase iPLA2γ is a major mediator releasing oxidized aliphatic chains from cardiolipin, integrating mitochondrial bioenergetics and signaling
Abstract
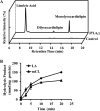
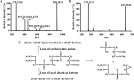
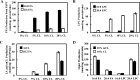
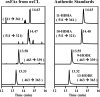
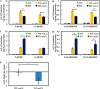
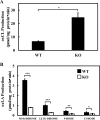
Cardiolipin (CL) is a dimeric phospholipid with critical roles in mitochondrial bioenergetics and signaling. Recently, inhibition of the release of oxidized fatty acyl chains from CL by the calcium-independent phospholipase A2γ (iPLA2γ)-selective inhibitor (R)-BEL suggested that iPLA2γ is responsible for the hydrolysis of oxidized CL and subsequent signaling mediated by the released oxidized fatty acids. However, chemical inhibition by BEL is subject to off-target pharmacologic effects. Accordingly, to unambiguously determine the role of iPLA2γ in the hydrolysis of oxidized CL, we compared alterations in oxidized CLs and the release of oxidized aliphatic chains from CL in experiments with purified recombinant iPLA2γ, germ-line iPLA2γ-/- mice, cardiac myocyte-specific iPLA2γ transgenic mice, and wild-type mice. Using charge-switch high mass accuracy LC-MS/MS with selected reaction monitoring and product ion accurate masses, we demonstrated that iPLA2γ is the major enzyme responsible for the release of oxidized aliphatic chains from CL. Our results also indicated that iPLA2γ selectively hydrolyzes 9-hydroxy-octadecenoic acid in comparison to 13-hydroxy-octadecenoic acid from oxidized CLs. Moreover, oxidative stress (ADP, NADPH, and Fe3+) resulted in the robust production of oxidized CLs in intact mitochondria from iPLA2γ-/- mice. In sharp contrast, oxidized CLs were readily hydrolyzed in mitochondria from wild-type mice during oxidative stress. Finally, we demonstrated that CL activates the iPLA2γ-mediated hydrolysis of arachidonic acid from phosphatidylcholine, thereby integrating the production of lipid messengers from different lipid classes in mitochondria. Collectively, these results demonstrate the integrated roles of CL and iPLA2γ in lipid second-messenger production and mitochondrial bioenergetics during oxidative stress.
Keywords: cardiolipin; lipid metabolism; lipid oxidation; lipid signaling; mass spectrometry (MS).
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

