Aspergillus fumigatus Trehalose-Regulatory Subunit Homolog Moonlights To Mediate Cell Wall Homeostasis through Modulation of Chitin Synthase Activity
- PMID: 28442603
- PMCID: PMC5405227
- DOI: 10.1128/mBio.00056-17
Aspergillus fumigatus Trehalose-Regulatory Subunit Homolog Moonlights To Mediate Cell Wall Homeostasis through Modulation of Chitin Synthase Activity
Abstract
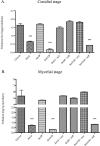
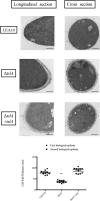
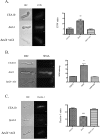
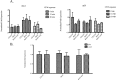
Trehalose biosynthesis is found in fungi but not humans. Proteins involved in trehalose biosynthesis are essential for fungal pathogen virulence in humans and plants through multiple mechanisms. Loss of canonical trehalose biosynthesis genes in the human pathogen Aspergillus fumigatus significantly alters cell wall structure and integrity, though the mechanistic link between these virulence-associated pathways remains enigmatic. Here we characterize genes, called tslA and tslB, which encode proteins that contain domains similar to those corresponding to trehalose-6-phosphate phosphatase but lack critical catalytic residues for phosphatase activity. Loss of tslA reduces trehalose content in both conidia and mycelia, impairs cell wall integrity, and significantly alters cell wall structure. To gain mechanistic insights into the role that TslA plays in cell wall homeostasis, immunoprecipitation assays coupled with liquid chromatography-tandem mass spectrometry (LC-MS/MS) were used to reveal a direct interaction between TslA and CsmA, a type V chitin synthase enzyme. TslA regulates not only chitin synthase activity but also CsmA sub-cellular localization. Loss of TslA impacts the immunopathogenesis of murine invasive pulmonary aspergillosis through altering cytokine production and immune cell recruitment. In conclusion, our data provide a novel model whereby proteins in the trehalose pathway play a direct role in fungal cell wall homeostasis and consequently impact fungus-host interactions.IMPORTANCE Human fungal infections are increasing globally due to HIV infections and increased use of immunosuppressive therapies for many diseases. Therefore, new antifungal drugs with reduced side effects and increased efficacy are needed to improve treatment outcomes. Trehalose biosynthesis exists in pathogenic fungi and is absent in humans. Components of the trehalose biosynthesis pathway are important for the virulence of human-pathogenic fungi, including Aspergillus fumigatus Consequently, it has been proposed that components of this pathway are potential targets for antifungal drug development. However, how trehalose biosynthesis influences the fungus-host interaction remains enigmatic. One phenotype associated with fungal trehalose biosynthesis mutants that remains enigmatic is cell wall perturbation. Here we discovered a novel moonlighting role for a regulatory-like subunit of the trehalose biosynthesis pathway in A. fumigatus that regulates cell wall homeostasis through modulation of chitin synthase localization and activity. As the cell wall is a current and promising therapeutic target for fungal infections, understanding the role of trehalose biosynthesis in cell wall homeostasis and virulence is expected to help define new therapeutic opportunities.
Keywords: Aspergillus fumigatus; cell wall; chitin; filamentous fungi; pathogenesis; trehalose.
Copyright © 2017 Thammahong et al.
Figures

References
-
- Patterson TF, Thompson GR III, Denning DW, Fishman JA, Hadley S, Herbrecht R, Kontoyiannis DP, Marr KA, Morrison VA, Nguyen MH, Segal BH, Steinbach WJ, Stevens DA, Walsh TJ, Wingard JR, Young JA, Bennett JE. 2016. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 63:e1–e60. doi: 10.1093/cid/ciw326. - DOI - PMC - PubMed
-
- Leloir LF, Cabib E. 1953. The enzymic synthesis of trehalose phosphate. J Am Chem Soc 75:5445–5446. doi: 10.1021/ja01117a528. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
