The Toll-Like Receptor 4 Antagonist Eritoran Protects Mice from Lethal Filovirus Challenge
- PMID: 28442605
- PMCID: PMC5405229
- DOI: 10.1128/mBio.00226-17
The Toll-Like Receptor 4 Antagonist Eritoran Protects Mice from Lethal Filovirus Challenge
Abstract
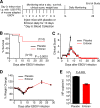
The 2013-2016 outbreak of Ebola virus (EBOV) in West Africa, which has seen intermittent reemergence since it was officially declared over in February of 2016, has demonstrated the need for the rapid development of therapeutic intervention strategies. Indirect evidence has suggested that the EBOV infection shares several commonalities associated with the onset of bacterial sepsis, including the development of a "cytokine storm." Eritoran, a Toll-like receptor 4 (TLR4) antagonist, was previously shown to result in protection of mice against lethal influenza virus infection. Here, we report that eritoran protects against the lethality caused by EBOV and the closely related Marburg virus (MARV) in mice. Daily administration of eritoran reduced clinical signs of the disease and, unexpectedly, resulted in reduced viral titers. Analysis of peripheral blood indicated that eritoran reduced granulocytosis despite an apparent increase in the percentage of activated neutrophils. Surprisingly, the increased survival rate and reduced viremia were not accompanied by increased CD3+ T lymphocytes, as lymphopenia was more pronounced in eritoran-treated mice. Overall, a global reduction in the levels of multiple cytokines, chemokines, and free radicals was detected in serum, suggesting that eritoran treatment may alleviate the severity of the "cytokine storm." Last, we provide compelling preliminary evidence suggesting that eritoran treatment may alter the kinetics of cytokine responses. Hence, these studies are the first to demonstrate the role of TLR4 in the pathogenesis of EBOV disease and indicate that eritoran is a prime candidate for further evaluation as a clinically viable therapeutic intervention strategy for EBOV and MARV infections.IMPORTANCE A hallmark of bacterial sepsis is the uncontrolled activation of the TLR4 pathway, which is the primary cause of the pathological features associated with this disease. Considering the importance of TLR4 signaling in bacterial sepsis and the remarkable pathological similarities associated with infections caused by filoviruses Ebola virus (EBOV) and Marburg virus (MARV), we assessed the ability of eritoran, a TLR4 antagonist, to protect mice against these viruses. Here, we show that eritoran effectively promotes survival of mice of filovirus infection, as 70% and 90% of mice receiving daily eritoran treatment survived lethal EBOV and MARV infections, respectively. Eritoran treatment resulted in a remarkable global reduction of inflammatory mediators, which is suggestive of the mechanism of action of this therapeutic treatment. These studies are the first to show the critical importance of the TLR4 pathway in the pathogenesis of filovirus infection and may provide a new avenue for therapeutic interventions.
Keywords: Ebola virus; Marburg virus; Toll-like receptor 4; cytokine storm; viral hemorrhagic fever.
Copyright © 2017 Younan et al.
Figures

References
-
- CDC 2016. Outbreaks chronology: Ebola virus disease. CDC, Atlanta, GA: https://www.cdc.gov/vhf/ebola/outbreaks/history/chronology.html.
-
- CDC 2016. Outbreak of Ebola in Guinea, Liberia, and Sierra Leone. CDC, Atlanta, GA: https://www.cdc.gov/vhf/ebola/outbreaks/2014-west-africa/index.html.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
