The EXIT Strategy: an Approach for Identifying Bacterial Proteins Exported during Host Infection
- PMID: 28442606
- PMCID: PMC5405230
- DOI: 10.1128/mBio.00333-17
The EXIT Strategy: an Approach for Identifying Bacterial Proteins Exported during Host Infection
Erratum in
-
Erratum for Perkowski et al., "The EXIT Strategy: an Approach for Identifying Bacterial Proteins Exported during Host Infection".mBio. 2017 Jun 20;8(3):e00872-17. doi: 10.1128/mBio.00872-17. mBio. 2017. PMID: 28634243 Free PMC article. No abstract available.
Abstract
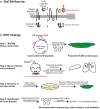
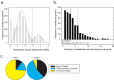
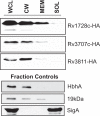
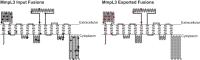
Exported proteins of bacterial pathogens function both in essential physiological processes and in virulence. Past efforts to identify exported proteins were limited by the use of bacteria growing under laboratory (in vitro) conditions. Thus, exported proteins that are exported only or preferentially in the context of infection may be overlooked. To solve this problem, we developed a genome-wide method, named EXIT (
Keywords: EXIT; Mycobacterium tuberculosis; beta-lactamase reporter; in vivo; membrane proteins; protein export; protein secretion; virulence.
Copyright © 2017 Perkowski et al.
Figures

Similar articles
-
Screening Mycobacterium tuberculosis Secreted Proteins Identifies Mpt64 as a Eukaryotic Membrane-Binding Bacterial Effector.mSphere. 2019 Jun 5;4(3):e00354-19. doi: 10.1128/mSphere.00354-19. mSphere. 2019. PMID: 31167949 Free PMC article.
-
Genome-wide identification of Mycobacterium tuberculosis exported proteins with roles in intracellular growth.J Bacteriol. 2011 Feb;193(4):854-61. doi: 10.1128/JB.01271-10. Epub 2010 Dec 10. J Bacteriol. 2011. PMID: 21148733 Free PMC article.
-
Beta-lactamase can function as a reporter of bacterial protein export during Mycobacterium tuberculosis infection of host cells.Microbiology (Reading). 2007 Oct;153(Pt 10):3350-3359. doi: 10.1099/mic.0.2007/008516-0. Microbiology (Reading). 2007. PMID: 17906134 Free PMC article.
-
The Sec Pathways and Exportomes of Mycobacterium tuberculosis.Microbiol Spectr. 2017 Apr;5(2). doi: 10.1128/microbiolspec.TBTB2-0013-2016. Microbiol Spectr. 2017. PMID: 28387178 Review.
-
The ins and outs of Mycobacterium tuberculosis protein export.Tuberculosis (Edinb). 2012 Mar;92(2):121-32. doi: 10.1016/j.tube.2011.11.005. Epub 2011 Dec 21. Tuberculosis (Edinb). 2012. PMID: 22192870 Free PMC article. Review.
Cited by
-
The cell envelope-associated phospholipid-binding protein LmeA is required for mannan polymerization in mycobacteria.J Biol Chem. 2017 Oct 20;292(42):17407-17417. doi: 10.1074/jbc.M117.804377. Epub 2017 Aug 29. J Biol Chem. 2017. PMID: 28855252 Free PMC article.
-
Role of LmeA, a Mycobacterial Periplasmic Protein, in Maintaining the Mannosyltransferase MptA and Its Product Lipomannan under Stress.mSphere. 2020 Nov 4;5(6):e01039-20. doi: 10.1128/mSphere.01039-20. mSphere. 2020. PMID: 33148829 Free PMC article.
-
The mycobacterial glycoside hydrolase LamH enables capsular arabinomannan release and stimulates growth.Nat Commun. 2024 Jul 9;15(1):5740. doi: 10.1038/s41467-024-50051-3. Nat Commun. 2024. PMID: 38982040 Free PMC article.
-
Protein Export into and across the Atypical Diderm Cell Envelope of Mycobacteria.Microbiol Spectr. 2019 Jul;7(4):10.1128/microbiolspec.gpp3-0043-2018. doi: 10.1128/microbiolspec.GPP3-0043-2018. Microbiol Spectr. 2019. PMID: 31400094 Free PMC article. Review.
-
Tracking Proteins Secreted by Bacteria: What's in the Toolbox?Front Cell Infect Microbiol. 2017 May 31;7:221. doi: 10.3389/fcimb.2017.00221. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28620586 Free PMC article. Review.
References
-
- McCann JR, Kurtz S, Braunstein M. 2009. Secreted and exported proteins important to Mycobacterium tuberculosis pathogenesis, p 265–298. In Wooldridge K (ed), Bacterial secreted proteins: secretory mechanisms and role in pathogenesis. Caister Academic Press, Norfolk, United Kingdom.
-
- Seo KS, Kim JW, Park JY, Viall AK, Minnich SS, Rohde HN, Schnider DR, Lim SY, Hong JB, Hinnebusch BJ, O’Loughlin JL, Deobald CF, Bohach GA, Hovde CJ, Minnich SA. 2012. Role of a new intimin/invasin-like protein in Yersinia pestis virulence. Infect Immun 80:3559–3569. doi:10.1128/IAI.00294-12. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

