Pharmacologic targeting ERK1/2 attenuates the development and progression of hyperuricemic nephropathy in rats
- PMID: 28442634
- PMCID: PMC5464913
- DOI: 10.18632/oncotarget.16995
Pharmacologic targeting ERK1/2 attenuates the development and progression of hyperuricemic nephropathy in rats
Abstract
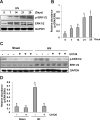
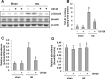
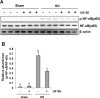
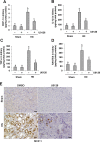
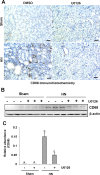
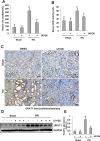
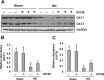
The pathogenesis of hyperuricemia-induced chronic kidney disease is largely unknown. In this study, we investigated whether extracellular signal-regulated kinases1/2 (ERK1/2) would contribute to the development of hyperuricemic nephropathy (HN). In a rat model of HN induced by feeding mixture of adenine and potassium oxonate, increased ERK1/2 phosphorylation and severe glomerular sclerosis and renal interstitial fibrosis were evident, in parallel with diminished levels of renal function and increased urine microalbumin excretion. Administration of U0126, which is a selective inhibitor of the ERK1/2 pathway, improved renal function, decreased urine microalbumin and inhibited activation of renal interstitial fibroblasts as well as accumulation of extracellular proteins. U0126 also inhibited hyperuricemia-induced expression of multiple profibrogenic cytokines/chemokines and infiltration of macrophages in the kidney. Furthermore, U0126 treatment suppressed xanthine oxidase, which mediates uric acid production. It also reduced expression of the urate anion exchanger 1, which promotes reabsorption of uric acid, and preserved expression of organic anion transporters 1 and 3, which accelerate uric acid excretion in the kidney of hyperuricemic rats. Finally, U0126 inhibited phosphorylation of Smad3, a key mediator in transforming growth factor (TGF-β) signaling. In cultured renal interstitial fibroblasts, inhibition of ERK1/2 activation by siRNA suppressed uric acid-induced activation of renal interstitial fibroblasts. Collectively, pharmacologic targeting of ERK1/2 can alleviate HN by suppressing TGF-β signaling, reducing inflammation responses, and inhibiting the molecular processes associated with elevation of blood uric acid levels in the body. Thus, ERK1/2 inhibition may be a potential approach for the prevention and treatment of hyperuricemic nephropathy.
Keywords: ERK1/2; TGF-β/Smad signaling pathway; hyperuricemic nephropathy; inflammation; urate transporters.
Conflict of interest statement
No authors have any competing interests.
Figures




Similar articles
-
EGF Receptor Inhibition Alleviates Hyperuricemic Nephropathy.J Am Soc Nephrol. 2015 Nov;26(11):2716-29. doi: 10.1681/ASN.2014080793. Epub 2015 Mar 18. J Am Soc Nephrol. 2015. PMID: 25788532 Free PMC article.
-
Natural flavonol fisetin attenuated hyperuricemic nephropathy via inhibiting IL-6/JAK2/STAT3 and TGF-β/SMAD3 signaling.Phytomedicine. 2021 Jul;87:153552. doi: 10.1016/j.phymed.2021.153552. Epub 2021 Mar 22. Phytomedicine. 2021. PMID: 33994251
-
Pharmacological inhibition of Src family kinases attenuates hyperuricemic nephropathy.Front Pharmacol. 2024 Mar 21;15:1352730. doi: 10.3389/fphar.2024.1352730. eCollection 2024. Front Pharmacol. 2024. PMID: 38576481 Free PMC article.
-
Mechanistic insights into hyperuricemia-associated renal abnormalities with special emphasis on epithelial-to-mesenchymal transition: Pathologic implications and putative pharmacologic targets.Pharmacol Res. 2020 Nov;161:105209. doi: 10.1016/j.phrs.2020.105209. Epub 2020 Sep 23. Pharmacol Res. 2020. PMID: 32979505 Review.
-
Uric Acid Elevation by Favipiravir, an Antiviral Drug.Tohoku J Exp Med. 2020 Jun;251(2):87-90. doi: 10.1620/tjem.251.87. Tohoku J Exp Med. 2020. PMID: 32536670 Review.
Cited by
-
A Review on Extracts, Chemical Composition and Product Development of Walnut Diaphragma Juglandis Fructus.Foods. 2023 Sep 8;12(18):3379. doi: 10.3390/foods12183379. Foods. 2023. PMID: 37761088 Free PMC article. Review.
-
Blockade of ERK1/2 by U0126 alleviates uric acid-induced EMT and tubular cell injury in rats with hyperuricemic nephropathy.Am J Physiol Renal Physiol. 2019 Apr 1;316(4):F660-F673. doi: 10.1152/ajprenal.00480.2018. Epub 2019 Jan 16. Am J Physiol Renal Physiol. 2019. PMID: 30648910 Free PMC article.
-
Induction of Fibrogenic Phenotype in Human Mesenchymal Stem Cells by Connective Tissue Growth Factor in a Hydrogel Model of Soft Connective Tissue.ACS Biomater Sci Eng. 2019 Sep 9;5(9):4531-4541. doi: 10.1021/acsbiomaterials.9b00425. Epub 2019 Jul 30. ACS Biomater Sci Eng. 2019. PMID: 33178886 Free PMC article.
-
Drug Transporters in the Kidney: Perspectives on Species Differences, Disease Status, and Molecular Docking.Front Pharmacol. 2021 Nov 29;12:746208. doi: 10.3389/fphar.2021.746208. eCollection 2021. Front Pharmacol. 2021. PMID: 34912216 Free PMC article. Review.
-
Recent advances on uric acid transporters.Oncotarget. 2017 Aug 10;8(59):100852-100862. doi: 10.18632/oncotarget.20135. eCollection 2017 Nov 21. Oncotarget. 2017. PMID: 29246027 Free PMC article. Review.
References
-
- Levey AS, Atkins R, Coresh J, Coresh J. Chronic kidney diseaseas a global public health problem: approaches and initiatives-aposition statement from Kidney Disease Improving Global Outcomes. Kidney Int. 2007;72:247–259. - PubMed
-
- Fried LF. Higher incidence of ESRD than mortality in theAASK study. J Am SocNephrol. 2010;21:1244–1246. - PubMed
-
- Sonoda H, Takase H, Dohi Y, Kimura G. Uric acid levels predict future development of chronic kidney disease. Am J Nephrol. 2011;33:352–357. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

