A novel missense mutation of Mip causes semi-dominant cataracts in the Nat mouse
- PMID: 28442635
- PMCID: PMC5543248
- DOI: 10.1538/expanim.17-0012
A novel missense mutation of Mip causes semi-dominant cataracts in the Nat mouse
Abstract
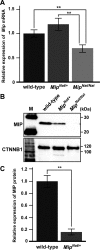
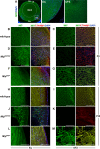
Major intrinsic protein of lens fiber (MIP) is one of the proteins essential for maintaining lens transparency while also contributing to dominant cataracts in humans. The Nodai cataract (Nat) mice harbor a spontaneous mutation in Mip and develop early-onset nuclear cataracts. The Nat mutation is a c.631G>A mutation (MipNat), resulting in a glycine-to-arginine substitution (p.Gly211Arg) in the sixth transmembrane domain. The MipNat/Nat homozygotes exhibit congenital cataracts caused by the degeneration of lens fiber cells. MIP normally localizes to the lens fiber cell membranes. However, the MipNat/Nat mice were found to lack an organelle-free zone, and the MIP was mislocalized to the nuclear membrane and perinuclear region. Furthermore, the MipNat/+ mice exhibited milder cataracts than MipNat/Nat mice due to the slight degeneration of the lens fiber cells. Although there were no differences in the localization of MIP to the membranes of lens fiber cells in MipNat/+ mice compared to that in wild-type mice, the protein levels of MIP were significantly reduced in the eyes. These findings suggest that cataractogenesis in MipNat mutants are caused by defects in MIP expression. Overall, the MipNat mice offer a novel model to better understand the phenotypes and mechanisms for the development of cataracts in patients that carry missense mutations in MIP.
Keywords: MIP; congenital cataract; missense mutation; mouse; semi-dominant cataract.
Figures




Similar articles
-
The relationship between major intrinsic protein genes and cataract.Int Ophthalmol. 2021 Jan;41(1):375-387. doi: 10.1007/s10792-020-01583-2. Epub 2020 Sep 12. Int Ophthalmol. 2021. PMID: 32920712 Review.
-
Identification and Functional Analysis of a Novel MIP Gene Mutation Associated with Congenital Cataract in a Chinese Family.PLoS One. 2015 May 6;10(5):e0126679. doi: 10.1371/journal.pone.0126679. eCollection 2015. PLoS One. 2015. PMID: 25946197 Free PMC article.
-
A novel MIP gene mutation analysis in a Chinese family affected with congenital progressive punctate cataract.PLoS One. 2014 Jul 17;9(7):e102733. doi: 10.1371/journal.pone.0102733. eCollection 2014. PLoS One. 2014. PMID: 25033405 Free PMC article.
-
Structural function of MIP/aquaporin 0 in the eye lens; genetic defects lead to congenital inherited cataracts.Handb Exp Pharmacol. 2009;(190):265-97. doi: 10.1007/978-3-540-79885-9_14. Handb Exp Pharmacol. 2009. PMID: 19096783 Review.
-
A novel MIP gene mutation associated with autosomal dominant congenital cataracts in a Chinese family.BMC Med Genet. 2014 Jan 9;15:6. doi: 10.1186/1471-2350-15-6. BMC Med Genet. 2014. PMID: 24405844 Free PMC article.
Cited by
-
Gender-Difference in Hair Length as Revealed by Crispr-Based Production of Long-Haired Mice with Dysfunctional FGF5 Mutations.Int J Mol Sci. 2022 Oct 6;23(19):11855. doi: 10.3390/ijms231911855. Int J Mol Sci. 2022. PMID: 36233155 Free PMC article.
-
A novel missense mutation in the gene encoding major intrinsic protein (MIP) in a Giant panda with unilateral cataract formation.BMC Genomics. 2021 Feb 2;22(1):100. doi: 10.1186/s12864-021-07386-8. BMC Genomics. 2021. PMID: 33530927 Free PMC article.
-
The relationship between major intrinsic protein genes and cataract.Int Ophthalmol. 2021 Jan;41(1):375-387. doi: 10.1007/s10792-020-01583-2. Epub 2020 Sep 12. Int Ophthalmol. 2021. PMID: 32920712 Review.
-
Genetic modifiers of rodent animal models: the role in cataractogenesis.Exp Anim. 2019 Nov 6;68(4):397-406. doi: 10.1538/expanim.19-0020. Epub 2019 May 20. Exp Anim. 2019. PMID: 31105106 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

