Cardioprotective effects of Cu(II)ATSM in human vascular smooth muscle cells and cardiomyocytes mediated by Nrf2 and DJ-1
- PMID: 28442712
- PMCID: PMC5431352
- DOI: 10.1038/s41598-016-0012-5
Cardioprotective effects of Cu(II)ATSM in human vascular smooth muscle cells and cardiomyocytes mediated by Nrf2 and DJ-1
Abstract
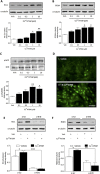
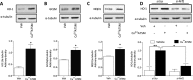
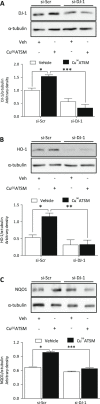
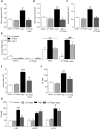
Cu(II)ATSM was developed as a hypoxia sensitive positron emission tomography agent. Recent reports have highlighted the neuroprotective properties of Cu(II)ATSM, yet there are no reports that it confers cardioprotection. We demonstrate that Cu(II)ATSM activates the redox-sensitive transcription factor Nrf2 in human coronary artery smooth muscle cells (HCASMC) and cardiac myocytes (HCM), leading to upregulation of antioxidant defense enzymes. Oral delivery of Cu(II)ATSM in mice induced expression of the Nrf2-regulated enzymes in the heart and aorta. In HCASMC, Cu(II)ATSM increased expression of the Nrf2 stabilizer DJ-1, and knockdown of Nrf2 or DJ-1 attenuated Cu(II)ATSM-mediated heme oxygenase-1 and NADPH quinone oxidoreductase-1 induction. Pre-treatment of HCASMC with Cu(II)ATSM protected against the pro-oxidant effects of angiotensin II (Ang II) by attenuating superoxide generation, apoptosis, proliferation and increases in intracellular calcium. Notably, Cu(II)ATSM-mediated protection against Ang II-induced HCASMC apoptosis was diminished by Nrf2 knockdown. Acute treatment with Cu(II)ATSM enhanced the association of DJ-1 with superoxide dismutase-1 (SOD1), paralleled by significant increases in intracellular Cu(II) levels and SOD1 activity. We describe a novel mechanism by which Cu(II)ATSM induces Nrf2-regulated antioxidant enzymes and protects against Ang II-mediated HCASMC dysfunction via activation of the Nrf2/DJ-1 axis. Cu(II)ATSM may provide a therapeutic strategy for cardioprotection via upregulation of antioxidant defenses.
Figures

Similar articles
-
Activation of Nrf2 contributes to the protective effect of Exendin-4 against angiotensin II-induced vascular smooth muscle cell senescence.Am J Physiol Cell Physiol. 2016 Oct 1;311(4):C572-C582. doi: 10.1152/ajpcell.00093.2016. Epub 2016 Aug 3. Am J Physiol Cell Physiol. 2016. PMID: 27488664
-
DJ-1 Mediates the Delayed Cardioprotection of Hypoxic Preconditioning Through Activation of Nrf2 and Subsequent Upregulation of Antioxidative Enzymes.J Cardiovasc Pharmacol. 2015 Aug;66(2):148-58. doi: 10.1097/FJC.0000000000000257. J Cardiovasc Pharmacol. 2015. PMID: 25915512
-
Activation of M3AChR (Type 3 Muscarinic Acetylcholine Receptor) and Nrf2 (Nuclear Factor Erythroid 2-Related Factor 2) Signaling by Choline Alleviates Vascular Smooth Muscle Cell Phenotypic Switching and Vascular Remodeling.Arterioscler Thromb Vasc Biol. 2020 Nov;40(11):2649-2664. doi: 10.1161/ATVBAHA.120.315146. Epub 2020 Sep 17. Arterioscler Thromb Vasc Biol. 2020. PMID: 32938216
-
Redox-sensitive transcription factor Nrf2 regulates vascular smooth muscle cell migration and neointimal hyperplasia.Arterioscler Thromb Vasc Biol. 2013 Apr;33(4):760-8. doi: 10.1161/ATVBAHA.112.300614. Epub 2013 Feb 14. Arterioscler Thromb Vasc Biol. 2013. PMID: 23413426
-
Nrf2/ARE regulated antioxidant gene expression in endothelial and smooth muscle cells in oxidative stress: implications for atherosclerosis and preeclampsia.Sheng Li Xue Bao. 2007 Apr 25;59(2):117-27. Sheng Li Xue Bao. 2007. PMID: 17437032 Review.
Cited by
-
Endothelium- and Fibroblast-Derived C-Type Natriuretic Peptide Prevents the Development and Progression of Aortic Aneurysm.Arterioscler Thromb Vasc Biol. 2025 Jul;45(7):1044-1063. doi: 10.1161/ATVBAHA.124.322350. Epub 2025 Apr 3. Arterioscler Thromb Vasc Biol. 2025. PMID: 40177775 Free PMC article.
-
Copper Preserves Vasculature Structure and Function by Protecting Endothelial Cells from Apoptosis in Ischemic Myocardium.J Cardiovasc Transl Res. 2021 Dec;14(6):1146-1155. doi: 10.1007/s12265-021-10128-6. Epub 2021 May 17. J Cardiovasc Transl Res. 2021. PMID: 33999373
-
Cu(ATSM) Increases P-Glycoprotein Expression and Function at the Blood-Brain Barrier in C57BL6/J Mice.Pharmaceutics. 2023 Aug 3;15(8):2084. doi: 10.3390/pharmaceutics15082084. Pharmaceutics. 2023. PMID: 37631298 Free PMC article.
-
Are Astrocytes the Predominant Cell Type for Activation of Nrf2 in Aging and Neurodegeneration?Antioxidants (Basel). 2017 Aug 18;6(3):65. doi: 10.3390/antiox6030065. Antioxidants (Basel). 2017. PMID: 28820437 Free PMC article. Review.
-
AITC induces MRP1 expression by protecting against CS/CSE-mediated DJ-1 protein degradation via activation of the DJ-1/Nrf2 axis.Korean J Physiol Pharmacol. 2020 Nov 1;24(6):481-492. doi: 10.4196/kjpp.2020.24.6.481. Korean J Physiol Pharmacol. 2020. PMID: 33093270 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

