Double-stranded DNA induces a prothrombotic phenotype in the vascular endothelium
- PMID: 28442771
- PMCID: PMC5430798
- DOI: 10.1038/s41598-017-01148-x
Double-stranded DNA induces a prothrombotic phenotype in the vascular endothelium
Abstract
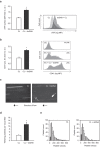
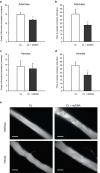
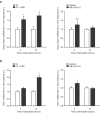
Double-stranded DNA (dsDNA) constitutes a potent activator of innate immunity, given its ability to bind intracellular pattern recognition receptors during viral infections or sterile tissue damage. While effects of dsDNA in immune cells have been extensively studied, dsDNA signalling and its pathophysiological implications in non-immune cells, such as the vascular endothelium, remain poorly understood. The aim of this study was to characterize prothrombotic effects of dsDNA in vascular endothelial cells. Transfection of cultured human endothelial cells with the synthetic dsDNA poly(dA:dT) induced upregulation of the prothrombotic molecules tissue factor and PAI-1, resulting in accelerated blood clotting in vitro, which was partly dependent on RIG-I signalling. Prothrombotic effects were also observed upon transfection of endothelial cells with hepatitis B virus DNA-containing immunoprecipitates as well human genomic DNA. In addition, dsDNA led to surface expression of von Willebrand factor resulting in increased platelet-endothelium-interactions under flow. Eventually, intrascrotal injection of dsDNA resulted in accelerated thrombus formation upon light/dye-induced endothelial injury in mouse cremaster arterioles and venules in vivo. In conclusion, we show that viral or endogenous dsDNA induces a prothrombotic phenotype in the vascular endothelium. These findings represent a novel link between pathogen- and danger-associated patterns within innate immunity and thrombosis.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

