Serotonin transporter deficiency drives estrogen-dependent obesity and glucose intolerance
- PMID: 28442777
- PMCID: PMC5430688
- DOI: 10.1038/s41598-017-01291-5
Serotonin transporter deficiency drives estrogen-dependent obesity and glucose intolerance
Abstract
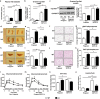
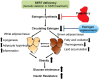
Depression and use of antidepressant medications are both associated with increased risk of obesity, potentially attributed to a reduced serotonin transporter (SERT) function. However, how SERT deficiency promotes obesity is unknown. Here, we demonstrated that SERT -/- mice display abnormal fat accumulation in both white and brown adipose tissues, glucose intolerance and insulin resistance while exhibiting suppressed aromatase (Cyp19a1) expression and reduced circulating 17β-estradiol levels. 17β-estradiol replacement in SERT -/- mice reversed the obesity and glucose intolerance, supporting a role for estrogen in SERT deficiency-associated obesity and glucose intolerance. Treatment of wild type mice with paroxetine, a chemical inhibitor of SERT, also resulted in Cyp19a1 suppression, decreased circulating 17β-estradiol levels, abnormal fat accumulation, and glucose intolerance. Such effects were not observed in paroxetine-treated SERT -/- mice. Conversely, pregnant SERT -/- mice displayed normalized estrogen levels, markedly reduced fat accumulation, and improved glucose tolerance, which can be eliminated by an antagonist of estrogen receptor α (ERα). Together, these findings support that estrogen suppression is involved in SERT deficiency-induced obesity and glucose intolerance, and suggest approaches to restore 17β-estradiol levels as a novel treatment option for SERT deficiency associated obesity and metabolic abnormalities.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





References
-
- Pratt, L. A., Brody, D. J. Depression and obesity in the U.S. adult household population, 2005–2010. NCHS Data Brief, 1–8 (2014). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

