Extreme Phenotype of Epidermal Growth Factor Receptor Inhibitor-induced Destructive Folliculitis
- PMID: 28442875
- PMCID: PMC5387879
- DOI: 10.4103/0974-7753.203174
Extreme Phenotype of Epidermal Growth Factor Receptor Inhibitor-induced Destructive Folliculitis
Abstract
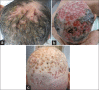
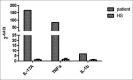
Due to the increasingly widespread use and side effect profile of epidermal growth factor receptor inhibitors (EGFRIs), cutaneous side effects of these drugs are frequently encountered. The EGFR is expressed on keratinocytes and fibroblasts. Inhibition of EGFR can produce a range of cutaneous adverse effects, the most frequent being a characteristic acneiform skin eruption. As the latter is associated with good anti-neoplastic responses, the onset of EGFRI-induced acneiform skin eruption is typically viewed as a positive sign by patients and physicians. It can usually be treated well with standard acne drugs, but in rare cases, the skin eruption can be so severe that systemic therapy and/or interruption of EGFRI treatment are required. One of the severest forms of EGFRI-induced skin eruption occurring on the head and neck area resembles folliculitis decalvans. Here, we discuss the management of such a case seen in our department. In addition, we present an analysis of tumor necrosis factor-α, interleukin-1β (IL-1β), and IL-17A expression based on immunohistochemical stains and qPCR.
Keywords: Epidermal growth factor receptor-inhibitor; folliculitis; folliculitis decalvans; interleukin-17A; interleukin-1β; tumor necrosis factor-α.
Conflict of interest statement
There are no conflicts of interest.
Figures

References
-
- Herbst RS. Review of epidermal growth factor receptor biology. Int J Radiat Oncol Biol Phys. 2004;59(2 Suppl):21–6. - PubMed
-
- Wollenberg A, Kroth J, Hauschild A, Dirschka T. Cutaneous side effects of EGFR inhibitors – Appearance and management. Dtsch Med Wochenschr. 2010;135:149–54. - PubMed
-
- Gresik EW, Kashimata M, Kadoya Y, Mathews R, Minami N, Yamashina S. Expression of epidermal growth factor receptor in fetal mouse submandibular gland detected by a biotinyltyramide-based catalyzed signal amplification method. J Histochem Cytochem. 1997;45:1651–7. - PubMed
-
- Rivera F, Vega-Villegas ME, Lopez-Brea MF, Marquez R. Current situation of panitumumab, matuzumab, nimotuzumab and zalutumumab. Acta Oncol. 2008;47:9–19. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

