Magnetic and pH dual-responsive mesoporous silica nanocomposites for effective and low-toxic photodynamic therapy
- PMID: 28442903
- PMCID: PMC5396969
- DOI: 10.2147/IJN.S127528
Magnetic and pH dual-responsive mesoporous silica nanocomposites for effective and low-toxic photodynamic therapy
Abstract
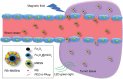
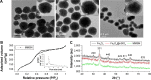
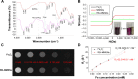
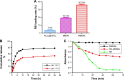
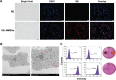
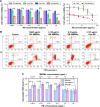
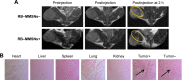
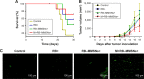
Nonspecific targeting, large doses and phototoxicity severely hamper the clinical effect of photodynamic therapy (PDT). In this work, superparamagnetic Fe3O4 mesoporous silica nanoparticles grafted by pH-responsive block polymer polyethylene glycol-b-poly(aspartic acid) (PEG-b-PAsp) were fabricated to load the model photosensitizer rose bengal (RB) in the aim of enhancing the efficiency of PDT. Compared to free RB, the nanocomposites (polyethylene glycol-b-polyaspartate-modified rose bengal-loaded magnetic mesoporous silica [RB-MMSNs]) could greatly enhance the cellular uptake due to their effective endocytosis by mouse melanoma B16 cell and exhibited higher induced apoptosis although with little dark toxicity. RB-MMSNs had little dark toxicity and even much could be facilitated by magnetic field in vitro. RB-MMSNs demonstrated 10 times induced apoptosis efficiency than that of free RB at the same RB concentration, both by cell counting kit-8 (CCK-8) result and apoptosis detection. Furthermore, RB-MMSNs-mediated PDT in vivo on tumor-bearing mice showed steady physical targeting of RB-MMSNs to the tumor site; tumor volumes were significantly reduced in the magnetic field with green light irradiation. More importantly, the survival time of tumor-bearing mice treated with RB-MMSNs was much prolonged. Henceforth, polyethylene glycol-b-polyaspartate-modified magnetic mesoporous silica (MMSNs) probably have great potential in clinical cancer photodynamic treatment because of their effective and low-toxic performance as photosensitizers' vesicles.
Keywords: magnetic mesoporous silica; magnetic targeting; pH responsive; photodynamic therapy; polymer polyethylene glycol-b-poly(aspartic acid); rose bengal.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures

References
-
- Fan W, Huang P, Chen X. Overcoming the Achilles’ heel of photodynamic therapy. Chem Soc Rev. 2016;45(23):6488–6519. - PubMed
-
- Inada NM, Kurachi C, Ferreira J, et al. Proc SPIE. 2009. Treatment of vulvar/vaginal condyloma by HPV: developed instrumentation and clinical report; p. 738054. (Photodynamic Therapy: Back to the Future).
-
- Atchison J, Kamila S, McEwan C, et al. Modulation of ROS production in photodynamic therapy using a pH controlled photoinduced electron transfer (PET) based sensitiser. Chem Commun (Camb) 2015;51(94):16832–16835. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

