Antibacterial activity and cytocompatibility of an implant coating consisting of TiO2 nanotubes combined with a GL13K antimicrobial peptide
- PMID: 28442908
- PMCID: PMC5396942
- DOI: 10.2147/IJN.S128775
Antibacterial activity and cytocompatibility of an implant coating consisting of TiO2 nanotubes combined with a GL13K antimicrobial peptide
Abstract
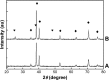
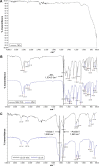
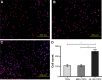
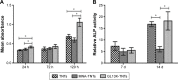
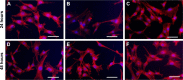
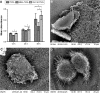
Prevention of implant-associated infections at an early stage of surgery is highly desirable for the long-term efficacy of implants in dentistry and orthopedics. Infection prophylaxis using conventional antibiotics is becoming less effective due to the development of bacteria resistant to multiple antibiotics. An ideal strategy to conquer bacterial infections is the local delivery of antibacterial agents. Therefore, antimicrobial peptide (AMP) eluting coatings on implant surfaces is a promising alternative. In this study, the feasibility of utilizing TiO2 nanotubes (TNTs), processed using anodization, as carriers to deliver a candidate AMP on titanium surfaces for the prevention of implant-associated infections is assessed. The broad-spectrum GL13K (GKIIKLKASLKLL-CONH2) AMP derived from human parotid secretory protein was selected and immobilized to TNTs using a simple soaking technique. Field emission scanning electron microscope, X-ray diffraction, Fourier transform infrared spectroscopy, and liquid chromatography-mass spectrometry analyses confirmed the successful immobilization of GL13K to anatase TNTs. The drug-loaded coatings demonstrated a sustained and slow drug release profile in vitro and eradicated the growth of Fusobacterium nucleatum and Porphyromonas gingivalis within 5 days of culture, as assessed by disk-diffusion assay. The GL13K-immobilized TNT (GL13K-TNT) coating demonstrated greater biocompatibility, compared with a coating produced by incubating TNTs with equimolar concentrations of metronidazole. GL13K-TNTs produced no observable cytotoxicity to preosteoblastic cells (MC3T3-E1). The coating may also have an immune regulatory effect, in support of rapid osseointegration around implants. Therefore, the combination of TNTs and AMP GL13K may achieve simultaneous antimicrobial and osteoconductive activities.
Keywords: antimicrobial peptide; nanotubes; orthopedic infections; titanium.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures



Similar articles
-
Bio-inspired stable antimicrobial peptide coatings for dental applications.Acta Biomater. 2013 Sep;9(9):8224-31. doi: 10.1016/j.actbio.2013.06.017. Epub 2013 Jun 19. Acta Biomater. 2013. PMID: 23791670 Free PMC article.
-
Biofunctionalization of microgroove titanium surfaces with an antimicrobial peptide to enhance their bactericidal activity and cytocompatibility.Colloids Surf B Biointerfaces. 2015 Apr 1;128:552-560. doi: 10.1016/j.colsurfb.2015.03.008. Epub 2015 Mar 7. Colloids Surf B Biointerfaces. 2015. PMID: 25800357
-
Local delivery of antimicrobial peptides using self-organized TiO2 nanotube arrays for peri-implant infections.J Biomed Mater Res A. 2012 Feb;100(2):278-85. doi: 10.1002/jbm.a.33251. Epub 2011 Nov 1. J Biomed Mater Res A. 2012. PMID: 22045618
-
Understanding and augmenting the stability of therapeutic nanotubes on anodized titanium implants.Mater Sci Eng C Mater Biol Appl. 2018 Jul 1;88:182-195. doi: 10.1016/j.msec.2018.03.007. Epub 2018 Mar 17. Mater Sci Eng C Mater Biol Appl. 2018. PMID: 29636134 Review.
-
Titania nanotube arrays for local drug delivery: recent advances and perspectives.Expert Opin Drug Deliv. 2015 Jan;12(1):103-27. doi: 10.1517/17425247.2014.945418. Epub 2014 Nov 7. Expert Opin Drug Deliv. 2015. PMID: 25376706 Review.
Cited by
-
Surface treatment strategies to combat implant-related infection from the beginning.J Orthop Translat. 2018 Sep 28;17:42-54. doi: 10.1016/j.jot.2018.09.001. eCollection 2019 Apr. J Orthop Translat. 2018. PMID: 31194031 Free PMC article. Review.
-
Nanomaterials in Dentistry: State of the Art and Future Challenges.Nanomaterials (Basel). 2020 Sep 7;10(9):1770. doi: 10.3390/nano10091770. Nanomaterials (Basel). 2020. PMID: 32906829 Free PMC article. Review.
-
Application of Antimicrobial Peptides on Biomedical Implants: Three Ways to Pursue Peptide Coatings.Int J Mol Sci. 2021 Dec 8;22(24):13212. doi: 10.3390/ijms222413212. Int J Mol Sci. 2021. PMID: 34948009 Free PMC article. Review.
-
Antimicrobial Peptides in the Battle against Orthopedic Implant-Related Infections: A Review.Pharmaceutics. 2021 Nov 12;13(11):1918. doi: 10.3390/pharmaceutics13111918. Pharmaceutics. 2021. PMID: 34834333 Free PMC article. Review.
-
Tetrahedral framework nucleic acids/hyaluronic acid-methacrylic anhydride hybrid hydrogel with antimicrobial and anti-inflammatory properties for infected wound healing.Int J Oral Sci. 2024 Apr 16;16(1):30. doi: 10.1038/s41368-024-00290-3. Int J Oral Sci. 2024. PMID: 38622128 Free PMC article.
References
-
- Derks J, Tomasi C. Peri-implant health and disease. A systematic review of current epidemiology. J Clin Periodontol. 2015;42(Suppl 16):S158–S171. - PubMed
-
- Darouiche RO. Device-associated infections: a macroproblem that starts with microadherence. Clin Infect Dis. 2001;33(9):1567–1572. - PubMed
-
- Eick S, Pfister W, Straube E. Antimicrobial susceptibility of anaerobic and capnophilic bacteria isolated from odontogenic abscesses and rapidly progressive periodontitis. Int J Antimicrob Agents. 1999;12(1):41–46. - PubMed
-
- Leonhardt Å, Renvert S, Dahlén G. Microbial findings at failing implants. Clin Oral Implants Res. 1999;10(5):339–345. - PubMed
-
- Jenkinson HF, Lamont RJ. Oral microbial communities in sickness and in health. Trends Microbiol. 2005;13(13):589–595. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

