EGF Enhances Oligodendrogenesis from Glial Progenitor Cells
- PMID: 28442994
- PMCID: PMC5387051
- DOI: 10.3389/fnmol.2017.00106
EGF Enhances Oligodendrogenesis from Glial Progenitor Cells
Abstract
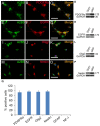
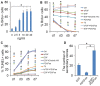
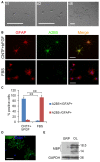
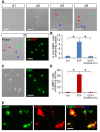
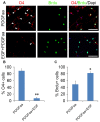
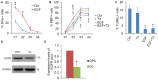
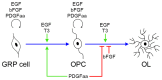
Emerging evidence indicates that epidermal growth factor (EGF) signaling plays a positive role in myelin development and repair, but little is known about its biological effects on the early generation and differentiation of oligodendrocyte (OL) lineage cells. In this study, we investigated the role of EGF in early OL development with isolated glial restricted precursor (GRP) cells. It was found that EGF collaborated with Platelet Derived Growth Factor-AA (PDGFaa) to promote the survival and self-renewal of GRP cells, but predisposed GRP cells to develop into O4- early-stage oligodendrocyte precursor cells (OPCs) in the absence of or PDGFaa. In OPCs, EGF synergized with PDGFaa to maintain their O4 negative antigenic phenotype. Upon PDGFaa withdrawal, EGF promoted the terminal differentiation of OPCs by reducing apoptosis and increasing the number of mature OLs. Together, these data revealed that EGF is an important mitogen to enhance oligodendroglial development.
Keywords: GRP cell; OPC; oligodendrocyte lineage; self-renewal; synergistic effect.
Figures
Similar articles
-
A Novel Approach for Amplification and Purification of Mouse Oligodendrocyte Progenitor Cells.Front Cell Neurosci. 2016 Aug 22;10:203. doi: 10.3389/fncel.2016.00203. eCollection 2016. Front Cell Neurosci. 2016. PMID: 27597818 Free PMC article.
-
The tripotential glial-restricted precursor (GRP) cell and glial development in the spinal cord: generation of bipotential oligodendrocyte-type-2 astrocyte progenitor cells and dorsal-ventral differences in GRP cell function.J Neurosci. 2002 Jan 1;22(1):248-56. doi: 10.1523/JNEUROSCI.22-01-00248.2002. J Neurosci. 2002. PMID: 11756508 Free PMC article.
-
Analysis of the spatial and morphological characteristics of oligodendrocytes from images of in vitro culture.MethodsX. 2024 Jun 8;13:102781. doi: 10.1016/j.mex.2024.102781. eCollection 2024 Dec. MethodsX. 2024. PMID: 38978971 Free PMC article.
-
Redox state as a central modulator of precursor cell function.Ann N Y Acad Sci. 2003 Jun;991:251-71. doi: 10.1111/j.1749-6632.2003.tb07481.x. Ann N Y Acad Sci. 2003. PMID: 12846992 Review.
-
Hormonal Regulation of Oligodendrogenesis I: Effects across the Lifespan.Biomolecules. 2021 Feb 14;11(2):283. doi: 10.3390/biom11020283. Biomolecules. 2021. PMID: 33672939 Free PMC article. Review.
Cited by
-
Function of B-Cell CLL/Lymphoma 11B in Glial Progenitor Proliferation and Oligodendrocyte Maturation.Front Mol Neurosci. 2018 Jan 24;11:4. doi: 10.3389/fnmol.2018.00004. eCollection 2018. Front Mol Neurosci. 2018. PMID: 29416501 Free PMC article.
-
Hypothermia combined with extracellular vesicles from clonally expanded immortalized mesenchymal stromal cells improves neurodevelopmental impairment in neonatal hypoxic-ischemic brain injury.J Neuroinflammation. 2023 Nov 27;20(1):280. doi: 10.1186/s12974-023-02961-0. J Neuroinflammation. 2023. PMID: 38012640 Free PMC article.
-
Serum NfL and EGFR/NfL ratio mRNAs as biomarkers for phenotype and disease severity of myelin oligodendrocyte glycoprotein IgG-associated disease.Front Immunol. 2024 May 14;15:1388734. doi: 10.3389/fimmu.2024.1388734. eCollection 2024. Front Immunol. 2024. PMID: 38807603 Free PMC article.
-
Distinct transcriptional changes distinguish efficient and poor remyelination in multiple sclerosis.Brain. 2025 Jun 3;148(6):2201-2217. doi: 10.1093/brain/awae414. Brain. 2025. PMID: 39718981 Free PMC article.
-
Innate Immune Pathways Promote Oligodendrocyte Progenitor Cell Recruitment to the Injury Site in Adult Zebrafish Brain.Cells. 2022 Feb 2;11(3):520. doi: 10.3390/cells11030520. Cells. 2022. PMID: 35159329 Free PMC article.
References
-
- Bansal R., Stefansson K., Pfeiffer S. E. (1992). Proligodendroblast antigen (POA), a developmental antigen expressed by A007/O4-positive oligodendrocyte progenitors prior to the appearance of sulfatide and galactocerebroside. J. Neurochem. 58, 2221–2229. 10.1111/j.1471-4159.1992.tb10967.x - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

