Cytosolic Glyceraldehyde-3-Phosphate Dehydrogenase Is Phosphorylated during Seed Development
- PMID: 28443115
- PMCID: PMC5387080
- DOI: 10.3389/fpls.2017.00522
Cytosolic Glyceraldehyde-3-Phosphate Dehydrogenase Is Phosphorylated during Seed Development
Abstract
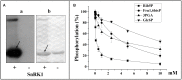
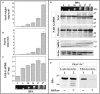
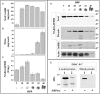
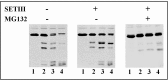
Cytosolic glyceraldehyde-3-phosphate dehydrogenase (NAD-GAPDH) is involved in a critical energetic step of glycolysis and also has many important functions besides its enzymatic activity. The recombinant wheat NAD-GAPDH was phosphorylated in vitro at Ser205 by a SNF1-Related protein kinase 1 (SnRK1) from wheat heterotrophic (but not from photosynthetic) tissues. The S205D mutant enzyme (mimicking the phosphorylated form) exhibited a significant decrease in activity but similar affinity toward substrates. Immunodetection and activity assays showed that NAD-GAPDH is phosphorylated in vivo, the enzyme depicting different activity, abundance and phosphorylation profiles during development of seeds that mainly accumulate starch (wheat) or lipids (castor oil seed). NAD-GAPDH activity gradually increases along wheat seed development, but protein levels and phosphorylation status exhibited slight changes. Conversely, in castor oil seed, the activity slightly increased and total protein levels do not significantly change in the first half of seed development but both abruptly decreased in the second part of development, when triacylglycerol synthesis and storage begin. Interestingly, phospho-NAD-GAPDH levels reached a maximum when the seed switch their metabolism to mainly support synthesis and accumulation of carbon reserves. After this point the castor oil seed NAD-GAPDH protein levels and activity highly decreased, and the protein stability assays showed that the protein would be degraded by the proteasome. The results presented herein suggest that phosphorylation of NAD-GAPDH during seed development would have impact on the partitioning of triose-phosphate between different metabolic pathways and cell compartments to support the specific carbon, energy and reducing equivalent demands during synthesis of storage products.
Keywords: castor oil seed; glyceraldehyde-3-phosphate; glycolysis; phosphorylation; seeds; wheat.
Figures




References
-
- Boyer P. D., Krebs E. G. (1986). The Enzymes: Control by Phosphorylation. Orlando, FL: Academic Press, Inc.
-
- Briarty L. G., Hughes C. E., Evers A. D. (1979). The developing endosperm of wheat-A stereological analysis. Ann. Bot. 44 641–658. 10.1093/oxfordjournals.aob.a085779 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

