Rubisco Activases: AAA+ Chaperones Adapted to Enzyme Repair
- PMID: 28443288
- PMCID: PMC5385338
- DOI: 10.3389/fmolb.2017.00020
Rubisco Activases: AAA+ Chaperones Adapted to Enzyme Repair
Abstract
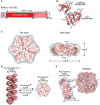
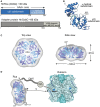
Ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco), the key enzyme of the Calvin-Benson-Bassham cycle of photosynthesis, requires conformational repair by Rubisco activase for efficient function. Rubisco mediates the fixation of atmospheric CO2 by catalyzing the carboxylation of the five-carbon sugar ribulose-1,5-bisphosphate (RuBP). It is a remarkably inefficient enzyme, and efforts to increase crop yields by bioengineering Rubisco remain unsuccessful. This is due in part to the complex cellular machinery required for Rubisco biogenesis and metabolic maintenance. To function, Rubisco must undergo an activation process that involves carboxylation of an active site lysine by a non-substrate CO2 molecule and binding of a Mg2+ ion. Premature binding of the substrate RuBP results in an inactive enzyme. Moreover, Rubisco can also be inhibited by a range of sugar phosphates, some of which are "misfire" products of its multistep catalytic reaction. The release of the inhibitory sugar molecule is mediated by the AAA+ protein Rubisco activase (Rca), which couples hydrolysis of ATP to the structural remodeling of Rubisco. Rca enzymes are found in the vast majority of photosynthetic organisms, from bacteria to higher plants. They share a canonical AAA+ domain architecture and form six-membered ring complexes but are diverse in sequence and mechanism, suggesting their convergent evolution. In this review, we discuss recent advances in understanding the structure and function of this important group of client-specific AAA+ proteins.
Keywords: AAA+ protein; CO2 fixation; Rubisco; Rubisco activase; photosynthesis.
Figures



References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

