Inhaled Nanoparticles Accumulate at Sites of Vascular Disease
- PMID: 28443337
- PMCID: PMC5444047
- DOI: 10.1021/acsnano.6b08551
Inhaled Nanoparticles Accumulate at Sites of Vascular Disease
Erratum in
-
Correction to"Inhaled Nanoparticles Accumulate at Sites of Vascular Disease".ACS Nano. 2017 Oct 24;11(10):10623-10624. doi: 10.1021/acsnano.7b06327. Epub 2017 Oct 4. ACS Nano. 2017. PMID: 28976185 Free PMC article. No abstract available.
Abstract
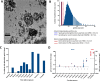
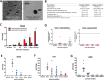
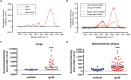
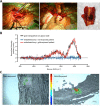
The development of engineered nanomaterials is growing exponentially, despite concerns over their potential similarities to environmental nanoparticles that are associated with significant cardiorespiratory morbidity and mortality. The mechanisms through which inhalation of nanoparticles could trigger acute cardiovascular events are emerging, but a fundamental unanswered question remains: Do inhaled nanoparticles translocate from the lung in man and directly contribute to the pathogenesis of cardiovascular disease? In complementary clinical and experimental studies, we used gold nanoparticles to evaluate particle translocation, permitting detection by high-resolution inductively coupled mass spectrometry and Raman microscopy. Healthy volunteers were exposed to nanoparticles by acute inhalation, followed by repeated sampling of blood and urine. Gold was detected in the blood and urine within 15 min to 24 h after exposure, and was still present 3 months after exposure. Levels were greater following inhalation of 5 nm (primary diameter) particles compared to 30 nm particles. Studies in mice demonstrated the accumulation in the blood and liver following pulmonary exposure to a broader size range of gold nanoparticles (2-200 nm primary diameter), with translocation markedly greater for particles <10 nm diameter. Gold nanoparticles preferentially accumulated in inflammation-rich vascular lesions of fat-fed apolipoproteinE-deficient mice. Furthermore, following inhalation, gold particles could be detected in surgical specimens of carotid artery disease from patients at risk of stroke. Translocation of inhaled nanoparticles into the systemic circulation and accumulation at sites of vascular inflammation provides a direct mechanism that can explain the link between environmental nanoparticles and cardiovascular disease and has major implications for risk management in the use of engineered nanomaterials.
Keywords: air pollution; atherosclerosis; cardiovascular; gold; nanoparticle; translocation.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Johnston H.; Pojana G.; Zuin S.; Jacobsen N. R.; Møller P.; Loft S.; Semmler-Behnke M.; McGuiness C.; Balharry D.; Marcomini A.; Wallin H.; Kreyling W.; Donaldson K.; Tran L.; Stone V. Engineered nanomaterial risk. Lessons learnt from completed nanotoxicology studies: potential solutions to current and future challenges. Crit. Rev. Toxicol. 2013, 43, 1–20. 10.3109/10408444.2012.738187. - DOI - PubMed
-
- World Health Organization. 7 million premature deaths annually linked to air pollution. http://www.who.int/mediacentre/news/releases/2014/air-pollution/en/. Accessed on March 29, 2017.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

