A unifying mathematical framework for experimental TCR-pMHC kinetic constants
- PMID: 28443634
- PMCID: PMC5405415
- DOI: 10.1038/srep46741
A unifying mathematical framework for experimental TCR-pMHC kinetic constants
Abstract
Receptor binding and triggering are central in Immunology as T cells activated through their T cell receptors (TCR) by protein antigens orchestrate immune responses. In order to understand receptor-ligand interactions, many groups working with different experimental techniques and assays have generated a vast body of knowledge during the last decades. However, in recent years a type of assays, referred to as two-dimensional or membrane-to-membrane, has questioned our current understanding of the role of different kinetic constants (for instance, on- versus off-rate constants) on TCR-ligand interaction and subsequent T cell activation. Here we present a general mathematical framework that provides a unifying umbrella to relate fundamental and effective (or experimentally determined) kinetic constants, as well as describe and compare state-of-the-art experimental methods. Our framework is able to predict the correlations between functional output, such as 1/EC50, and effective kinetic constants for a range of different experimental assays (in two and three dimensions). Furthermore, our approach can be applied beyond Immunology, and serve as a "translation method" for the biochemical characterization of receptor-ligand interactions.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

 , E1;
, E1;  , V-OVA;
, V-OVA;  , R4.
, R4.
 ,
,  for diffusion/encounter, e+, e− for rotation/orientation and k+, k− for binding and unbinding, respectively. The fundamental kinetic constants are defined in Table 1.
for diffusion/encounter, e+, e− for rotation/orientation and k+, k− for binding and unbinding, respectively. The fundamental kinetic constants are defined in Table 1.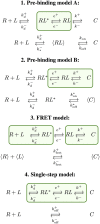
 ,
,  ,
,  and
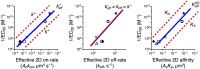
and  , as determined with the AF assay and the SS model. The red lines correspond to the fundamental binding constants (k+, k− and KA). A dashed red line indicates an uncertainty in the direction and magnitude of the shift corresponding to a given fundamental binding parameter, depending on whether
, as determined with the AF assay and the SS model. The red lines correspond to the fundamental binding constants (k+, k− and KA). A dashed red line indicates an uncertainty in the direction and magnitude of the shift corresponding to a given fundamental binding parameter, depending on whether  is positive or negative and whether
is positive or negative and whether  is relatively large or small. See details in Cases 1, 2 and 4.
is relatively large or small. See details in Cases 1, 2 and 4.
 ,
,  , and
, and  from the AF assay. The cyan lines are the predicted trend lines for the correlation between log(1/EC50) and the logarithm of the effective parameters
from the AF assay. The cyan lines are the predicted trend lines for the correlation between log(1/EC50) and the logarithm of the effective parameters  ,
,  , and
, and  from the 2D FRET assay, as inferred using the results in Cases 1 and 3 and further discussed in Case 4. Dashed vertical lines are drawn to help visualize the asymptotic limits of
from the 2D FRET assay, as inferred using the results in Cases 1 and 3 and further discussed in Case 4. Dashed vertical lines are drawn to help visualize the asymptotic limits of  and
and  . Their particular positions, as well as that of cyan lines, are for illustrative purposes.
. Their particular positions, as well as that of cyan lines, are for illustrative purposes.
 ,
,  , and
, and  from the SPR assay. The cyan lines are the predicted trend lines for the correlation between log(1/EC50) and the logarithm of the effective parameters
from the SPR assay. The cyan lines are the predicted trend lines for the correlation between log(1/EC50) and the logarithm of the effective parameters  ,
,  , and
, and  from the 3D FRET assay, as inferred using the results in Case 5. Dashed vertical lines are drawn to help visualize the asymptotic limits of
from the 3D FRET assay, as inferred using the results in Case 5. Dashed vertical lines are drawn to help visualize the asymptotic limits of  and
and  . Their particular positions, as well as that of cyan lines, are for illustrative purposes.
. Their particular positions, as well as that of cyan lines, are for illustrative purposes.References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

