Allogeneic Human Mesenchymal Stem Cell Infusions for Aging Frailty
- PMID: 28444181
- PMCID: PMC5861970
- DOI: 10.1093/gerona/glx056
Allogeneic Human Mesenchymal Stem Cell Infusions for Aging Frailty
Abstract
Background: Impaired endogenous stem cell repair capacity is hypothesized to be a biologic basis of frailty. Therapies that restore regenerative capacity may therefore be beneficial. This Phase 1 study evaluated the safety and potential efficacy of intravenous, allogeneic, human mesenchymal stem cell (allo-hMSC)-based therapy in patients with aging frailty.
Methods: In this nonrandomized, dose-escalation study, patients received a single intravenous infusion of allo-hMSCs: 20-million (n = 5), 100-million (n = 5), or 200-million cells (n = 5). The primary endpoint was incidence of any treatment-emergent serious adverse events measured at 1 month postinfusion. The secondary endpoints were functional efficacy domains and inflammatory biomarkers, measured at 3 and 6 months, respectively.
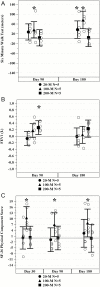
Results: There were no treatment-emergent serious adverse events at 1-month postinfusion or significant donor-specific immune reactions during the first 6 months. There was one death at 258 days postinfusion in the 200-million group. In all treatment groups, 6-minute walk distance increased at 3 months (p = .02) and 6 months (p = .001) and TNF-α levels decreased at 6 months (p < .0001). Overall, the 100-million dose showed the best improvement in all parameters, with the exception of TNF-α, which showed an improvement in both the 100- and 200-million groups (p = .0001 and p = .0001, respectively). The 100-million cell-dose group also showed significant improvements in the physical component of the SF-36 quality of life assessment at all time points relative to baseline.
Conclusions: Allo-hMSCs are safe and immunologically tolerated in aging frailty patients. Improvements in functional and immunologic status suggest that ongoing clinical development of cell-based therapy is warranted for frailty.
Keywords: Cell-based therapy; Inflammation; Physical function; Regenerative medicine.
© The Author 2017. Published by Oxford University Press on behalf of The Gerontological Society of America. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures


References
-
- Collard RM, Boter H, Schoevers RA, Oude Voshaar RC. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc. 2012;60:1487–1492. doi:10.1111/j.1532-5415.2012.04054.x - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

