Multiple Aggregation Pathways in Human γS-Crystallin and Its Aggregation-Prone G18V Variant
- PMID: 28444328
- PMCID: PMC5407245
- DOI: 10.1167/iovs.16-20621
Multiple Aggregation Pathways in Human γS-Crystallin and Its Aggregation-Prone G18V Variant
Abstract
Purpose: Cataract results from the formation of light-scattering precipitates due to point mutations or accumulated damage in the structural crystallins of the eye lens. Although excised cataracts are predominantly amorphous, in vitro studies show that crystallins are capable of adopting a variety of morphologies depending on the preparation method. Here we characterize thermal, pH-dependent, and UV-irradiated aggregates from wild-type human γS-crystallin (γS-WT) and its aggregation-prone variant, γS-G18V.
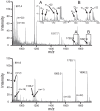
Methods: Aggregates of γS-WT and γS-G18V were prepared under acidic, neutral, and basic pH conditions and held at 25°C or 37°C for 48 hours. UV-induced aggregates were produced by irradiation with a 355-nm laser. Aggregation and fibril formation were monitored via turbidity and thioflavin T (ThT) assays. Aggregates were characterized using intrinsic aromatic fluorescence, powder x-ray diffraction, and mass spectrometry.
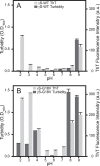
Results: γS-crystallin aggregates displayed different characteristics depending on the preparation method. γS-G18V produced a larger amount of detectable aggregates than did γS-WT and at less-extreme conditions. Aggregates formed under basic and acidic conditions yielded elevated ThT fluorescence; however, aggregates formed at low pH did not produce strongly turbid solutions. UV-induced aggregates produced highly turbid solutions but displayed only moderate ThT fluorescence. X-ray diffraction confirms amyloid character in low-pH samples and UV-irradiated samples, although the relative amounts vary.
Conclusions: γS-G18V demonstrates increased aggregation propensity compared to γS-WT when treated with heat, acid, or UV light. The resulting aggregates differ in their ThT fluorescence and turbidity, suggesting that at least two different aggregation pathways are accessible to both proteins under the conditions tested.
Figures




Similar articles
-
Function and Aggregation in Structural Eye Lens Crystallins.Acc Chem Res. 2020 Apr 21;53(4):863-874. doi: 10.1021/acs.accounts.0c00014. Epub 2020 Apr 9. Acc Chem Res. 2020. PMID: 32271004 Free PMC article. Review.
-
Human αB-crystallin discriminates between aggregation-prone and function-preserving variants of a client protein.Biochim Biophys Acta Gen Subj. 2020 Mar;1864(3):129502. doi: 10.1016/j.bbagen.2019.129502. Epub 2019 Dec 5. Biochim Biophys Acta Gen Subj. 2020. PMID: 31812542 Free PMC article.
-
The G18V CRYGS mutation associated with human cataracts increases gammaS-crystallin sensitivity to thermal and chemical stress.Biochemistry. 2009 Aug 4;48(30):7334-41. doi: 10.1021/bi900467a. Biochemistry. 2009. PMID: 19558189 Free PMC article.
-
Increased hydrophobic surface exposure in the cataract-related G18V variant of human γS-crystallin.Biochim Biophys Acta. 2016 Jan;1860(1 Pt B):325-32. doi: 10.1016/j.bbagen.2015.09.022. Epub 2015 Oct 14. Biochim Biophys Acta. 2016. PMID: 26459004 Free PMC article.
-
The βγ-crystallins: native state stability and pathways to aggregation.Prog Biophys Mol Biol. 2014 Jul;115(1):32-41. doi: 10.1016/j.pbiomolbio.2014.05.002. Epub 2014 May 14. Prog Biophys Mol Biol. 2014. PMID: 24835736 Free PMC article. Review.
Cited by
-
Development for Probiotics Based Insulin Delivery System.Curr Issues Mol Biol. 2025 Feb 21;47(3):137. doi: 10.3390/cimb47030137. Curr Issues Mol Biol. 2025. PMID: 40136391 Free PMC article.
-
Mercury-induced aggregation of human lens γ-crystallins reveals a potential role in cataract disease.J Biol Inorg Chem. 2018 Oct;23(7):1105-1118. doi: 10.1007/s00775-018-1607-z. Epub 2018 Aug 30. J Biol Inorg Chem. 2018. PMID: 30167892
-
Function and Aggregation in Structural Eye Lens Crystallins.Acc Chem Res. 2020 Apr 21;53(4):863-874. doi: 10.1021/acs.accounts.0c00014. Epub 2020 Apr 9. Acc Chem Res. 2020. PMID: 32271004 Free PMC article. Review.
-
Human γS-Crystallin-Copper Binding Helps Buffer against Aggregation Caused by Oxidative Damage.Biochemistry. 2020 Jun 30;59(25):2371-2385. doi: 10.1021/acs.biochem.0c00293. Epub 2020 Jun 12. Biochemistry. 2020. PMID: 32510933 Free PMC article.
-
Chemical Properties Determine Solubility and Stability in βγ-Crystallins of the Eye Lens.Chembiochem. 2021 Apr 16;22(8):1329-1346. doi: 10.1002/cbic.202000739. Epub 2021 Feb 10. Chembiochem. 2021. PMID: 33569867 Free PMC article. Review.
References
-
- Delaye M,, Tardieu A. Short-range order of crystallin proteins accounts for eye lens transparency. Nature. 1983; 302: 415–417. - PubMed
-
- Tardieu A,, Vérétout F,, Krop B,, Slingsby C. Protein interactions in the calf eye lens: interactions between beta-crystallins are repulsive whereas in gamma-crystallins they are attractive. Eur Biophys J. 1992; 21: 1–12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

