Selective progesterone receptor modulators (SPRMs) for uterine fibroids
- PMID: 28444736
- PMCID: PMC6478099
- DOI: 10.1002/14651858.CD010770.pub2
Selective progesterone receptor modulators (SPRMs) for uterine fibroids
Abstract
Background: Uterine fibroids are smooth muscle tumours arising from the uterus. These tumours, although benign, are commonly associated with abnormal uterine bleeding, bulk symptoms and reproductive dysfunction. The importance of progesterone in fibroid pathogenesis supports selective progesterone receptor modulators (SPRMs) as effective treatment. Both biochemical and clinical evidence suggests that SPRMs may reduce fibroid growth and ameliorate symptoms. SPRMs can cause unique histological changes to the endometrium that are not related to cancer, are not precancerous and have been found to be benign and reversible. This review summarises randomised trials conducted to evaluate the effectiveness of SPRMs as a class of medication for treatment of individuals with fibroids.
Objectives: To evaluate the effectiveness and safety of SPRMs for treatment of premenopausal women with uterine fibroids.
Search methods: We searched the Specialised Register of the Cochrane Gynaecology and Fertility Group, the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, PsycINFO, the Cumulative Index to Nursing and Allied Health Literature (CINAHL) and clinical trials registries from database inception to May 2016. We handsearched the reference lists of relevant articles and contacted experts in the field to request additional data.
Selection criteria: Included studies were randomised controlled trials (RCTs) of premenopausal women with fibroids who were treated for at least three months with a SPRM.
Data collection and analysis: Two review authors independently reviewed all eligible studies identified by the search. We extracted data and assessed risk of bias independently using standard forms. We analysed data using mean differences (MDs) or standardised mean differences (SMDs) for continuous data and odds ratios (ORs) for dichotomous data. We performed meta-analyses using the random-effects model. Our primary outcome was change in fibroid-related symptoms.
Main results: We included in the review 14 RCTs with a total of 1215 study participants. We could not extract complete data from three studies. We included in the meta-analysis 11 studies involving 1021 study participants: 685 received SPRMs and 336 were given a control intervention (placebo or leuprolide). Investigators evaluated three SPRMs: mifepristone (five studies), ulipristal acetate (four studies) and asoprisnil (two studies). The primary outcome was change in fibroid-related symptoms (symptom severity, health-related quality of life, abnormal uterine bleeding, pelvic pain). Adverse event reporting in the included studies was limited to SPRM-associated endometrial changes. More than half (8/14) of these studies were at low risk of bias in all domains. The most common limitation of the other studies was poor reporting of methods. The main limitation for the overall quality of evidence was potential publication bias. SPRM versus placebo SPRM treatment resulted in improvements in fibroid symptom severity (MD -20.04 points, 95% confidence interval (CI) -26.63 to -13.46; four RCTs, 171 women, I2 = 0%; moderate-quality evidence) and health-related quality of life (MD 22.52 points, 95% CI 12.87 to 32.17; four RCTs, 200 women, I2 = 63%; moderate-quality evidence) on the Uterine Fibroid Symptom Quality of Life Scale (UFS-QoL, scale 0 to 100). Women treated with an SPRM showed reduced menstrual blood loss on patient-reported bleeding scales, although this effect was small (SMD -1.11, 95% CI -1.38 to -0.83; three RCTs, 310 women, I2 = 0%; moderate-quality evidence), along with higher rates of amenorrhoea (29 per 1000 in the placebo group vs 237 to 961 per 1000 in the SPRM group; OR 82.50, 95% CI 37.01 to 183.90; seven RCTs, 590 women, I2 = 0%; moderate-quality evidence), compared with those given placebo. We could draw no conclusions regarding changes in pelvic pain owing to variability in the estimates. With respect to adverse effects, SPRM-associated endometrial changes were more common after SPRM therapy than after placebo (OR 15.12, 95% CI 6.45 to 35.47; five RCTs, 405 women, I2 = 0%; low-quality evidence). SPRM versus leuprolide acetate In comparing SPRM versus other treatments, two RCTs evaluated SPRM versus leuprolide acetate. One RCT reported primary outcomes. No evidence suggested a difference between SPRM and leuprolide groups for improvement in quality of life, as measured by UFS-QoL fibroid symptom severity scores (MD -3.70 points, 95% CI -9.85 to 2.45; one RCT, 281 women; moderate-quality evidence) and health-related quality of life scores (MD 1.06 points, 95% CI -5.73 to 7.85; one RCT, 281 women; moderate-quality evidence). It was unclear whether results showed a difference between SPRM and leuprolide groups for reduction in menstrual blood loss based on the pictorial blood loss assessment chart (PBAC), as confidence intervals were wide (MD 6 points, 95% CI -40.95 to 50.95; one RCT, 281 women; low-quality evidence), or for rates of amenorrhoea (804 per 1000 in the placebo group vs 732 to 933 per 1000 in the SPRM group; OR 1.14, 95% CI 0.60 to 2.16; one RCT, 280 women; moderate-quality evidence). No evidence revealed differences between groups in pelvic pain scores based on the McGill Pain Questionnaire (scale 0 to 45) (MD -0.01 points, 95% CI -2.14 to 2.12; 281 women; moderate-quality evidence). With respect to adverse effects, SPRM-associated endometrial changes were more common after SPRM therapy than after leuprolide treatment (OR 10.45, 95% CI 5.38 to 20.33; 301 women; moderate-quality evidence).
Authors' conclusions: Short-term use of SPRMs resulted in improved quality of life, reduced menstrual bleeding and higher rates of amenorrhoea than were seen with placebo. Thus, SPRMs may provide effective treatment for women with symptomatic fibroids. Evidence derived from one RCT showed no difference between leuprolide acetate and SPRM with respect to improved quality of life and bleeding symptoms. Evidence was insufficient to show whether effectiveness was different between SPRMs and leuprolide. Investigators more frequently observed SPRM-associated endometrial changes in women treated with SPRMs than in those treated with placebo or leuprolide acetate. As noted above, SPRM-associated endometrial changes are benign, are not related to cancer and are not precancerous. Reporting bias may impact the conclusion of this meta-analysis. Well-designed RCTs comparing SPRMs versus other treatments are needed.
Conflict of interest statement
AM participated in a speakers bureau or served on the advisory board for Allergan (manufacturer of ulipristal acetate in Canada), Abbvie (manufacturer of leuprolide), Bayer, Hologic and Medtronic.
MS, TC and LW have no conflicts of interest to declare.
Figures
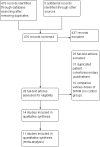
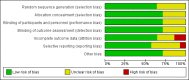
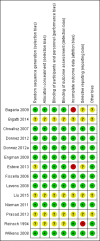






Update of
References
References to studies included in this review
Bagaria 2009 {published data only}
-
- Bagaria M, Suneja A, Vaid NB, Guleria K, Mishra K. Low‐dose mifepristone in treatment of uterine leiomyoma: a randomised double‐blind placebo‐controlled clinical trial. Australian and New Zealand Journal of Obstetrics and Gynaecology 2009;49:77‐83. - PubMed
Bigatti 2014 {published data only}
-
- Bigatti G, Lemmello R, Desgro M, Pollino S, Santirocco M. Progesterone preoperative treatment for myomectomy with the intrauterine bigatti shaver (IBS). Gynecological Surgery 2014;1:119‐20.
Chwalisz 2007 {published data only}
-
- Chwalisz K, Larsen L, Edmonds A, Winkel C. Effectiveness of asoprisnil, a selective progesterone receptor modulator (SPRM) in treating uterine leiomyomata. Advances in Uterine Leiomyoma Research: 2nd NIH International Congress. 2005.
-
- Chwalisz K, Larsen L, Mattia‐Goldberg C, Edmonds A, Elger W, Winkel CA. A randomized, controlled trial of asoprisnil, a novel selective progesterone receptor modulator, in women with uterine leiomyomata. Fertility and Sterility 2007;87:1399‐412. - PubMed
-
- Chwalisz K, Larsen L, McCrary K, Edmonds A. Effects of the novel selective progesterone receptor modulator (SPRM) asoprisnil on bleeding patterns in subjects with leiomyomata. Journal of the Society for Gynecologic Investigation 2004;11:320A‐1A.
-
- Larsen L, Coyne K, Chwalisz K. Validation of the menstrual pictogram in women with leiomyomata associated with heavy menstrual bleeding. Reproductive Sciences (Thousand Oaks, Calif.) 2013;20:680‐7. - PubMed
Donnez 2012 {published data only}
-
- Barlow DH, Lumsden MA, Fauser BCJM, Terrill P, Bestel E. Individualized vaginal bleeding experience of women with uterine fibroids in the PEARL I randomized controlled trial comparing the effects of ulipristal acetate or placebo. Human Reproduction 2014;29:480‐9. - PubMed
-
- Donnez J, Tatarchuk TF, Bouchard P, Puscasiu L, Zakharenko NF, Ivanova T, et al. Ulipristal acetate versus placebo for fibroid treatment before surgery. New England Journal of Medicine 2012;366:409‐20. - PubMed
-
- Williams AR, Bergeron C, Barlow DH, Ferenczy A. Endometrial morphology after treatment of uterine fibroids with the selective progesterone receptor modulator, ulipristal acetate. International Journal of Gynecological Pathology 2012;31:556‐69. - PubMed
Donnez 2012a {published data only}
-
- Donnez J, Tomaszewski J, Vazquez F, Bouchard P, Lemieszczuk B, Baro F, et al. Ulipristal acetate versus leuprolide acetate for uterine fibroids. New England Journal of Medicine 2012;366:421‐32. - PubMed
Engman 2009 {published data only}
-
- Engman M, Granberg S, Williams AR, Meng CX, Lalitkumar PG, Gemzell‐Danielsson K. Mifepristone for treatment of uterine leiomyoma. A prospective randomized placebo controlled trial. Human Reproduction 2009;24:1870‐9. - PubMed
Esteve 2013 {published data only}
Fiscella 2006 {published data only}
-
- Eisinger SH, Fiscella K, Meldrum S, Feng C, Fisher S, Guzick DS. Effect of mifepristone on quality of life for women with symptomatic fibroids. Fertility and Sterility 2006;86:S41‐2.
-
- Fiscella K, Eisinger SH, Meldrum S, Feng C, Fisher SG, Guzick DS. Effect of mifepristone for symptomatic leiomyomata on quality of life and uterine size: a randomized controlled trial. Obstetrics and Gynecology 2006;108:1381‐7. - PubMed
Levens 2008 {published data only}
-
- Levy G, Avila N, Armstrong AY, Nieman L. Does the selective progesterone receptor modulator ulipristal normalize the uterine cavity in women with leiomyoma. Reproductive Sciences 2011;1:95A.
-
- Segal TR, Zarek SM, Mumford SL, Plowden TC, Nieman LK, Segars JH, et al. Radiographic and histopathologic endometrial characteristics of women undergoing treatment with ulipristal acetate (UPA). Fertility and Sterility 2014;102:e286.
Liu 2015 {published data only}
-
- Liu C. Low‐dose mifepristone versus placebo to treat uterine myoma: a double‐blind, randomized clinical trial. Journal of Minimally Invasive Gynecology 2015;22(6):S91. - PubMed
Nieman 2011 {published data only}
-
- Fru KN, Levy G, Wesley R, Neiman L, Venkatesan A, Armstrong A. Prolactin as a biomarker for tumor burden and response to UPA therapy in African American women with symptomatic leiomyoma. Reproductive Sciences 2013;20:150A.
-
- Green L J, Levy G, Wesley R, Nieman L, Armstrong A. Efficacy of ulipristal acetate for the treatment of symptomatic uterine leiomyomas in African Americans. Fertility and Sterility 2013;98:S96.
-
- Levy G, Avila N, Armstrong AY, Nieman L. Does the selective progesterone receptor modulator ulipristal normalize the uterine cavity in women with leiomyoma. Reproductive Sciences 2011;1:95A.
-
- Nieman L, Chabbert‐Buffet N, Bouchard P. Endocrine safety of ulipristal acetate, a selective progesterone receptor modulator (SPRM): results from two phase II randomised, placebo‐controlled studies. Endocrine Abstracts 2010;22:P480.
Prasad 2013 {published data only}
-
- Prasad S, Varun N, Kumar A. Effect of low dose mifepristone on uterine leiomyoma in reproductive age group. Fertility and Sterility 2013;3:S78.
Reinsch 1994 {published data only}
-
- Reinsch RC, Murphy AA, Morales AJ, Yen SS. The effects of RU 486 and leuprolide acetate on uterine artery blood flow in the fibroid uterus: a prospective, randomized study. American Journal of Obstetrics and Gynecology 1994;170:1623‐7; discussion 1627‐8. - PubMed
Wilkens 2008 {published data only}
-
- Larsen L, Coyne K, Chwalisz K. Validation of the menstrual pictogram in women with leiomyomata associated with heavy menstrual bleeding. Reproductive Sciences 2013;20:680‐7. - PubMed
-
- Wilkens J, Chwalisz K, Han C, Walker J, Cameron IT, Ingamells S, et al. Effects of the selective progesterone receptor modulator asoprisnil on uterine artery blood flow, ovarian activity, and clinical symptoms in patients with uterine leiomyomata scheduled for hysterectomy. Journal of Clinical Endocrinology and Metabolism 2008;93:4664‐71. - PubMed
-
- Wilkens J, Williams AR, Chwalisz K, Han C, Cameron IT, Critchley HO. Effect of asoprisnil on uterine proliferation markers and endometrial expression of the tumour suppressor gene, PTEN. Human Reproduction 2009;24:1036‐44. - PubMed
-
- Williams AR, Critchley HO, Osei J, Ingamells S, Cameron IT, Han C, et al. The effects of the selective progesterone receptor modulator asoprisnil on the morphology of uterine tissues after 3 months treatment in patients with symptomatic uterine leiomyomata. Human Reproduction 2007;22:1696‐704. - PubMed
References to studies excluded from this review
Carbonell 2008 {published data only}
-
- Carbonell Esteve JL, Acosta R, Heredia B, Perez Y, Castaneda MC, Hernandez AV. Mifepristone for the treatment of uterine leiomyomas: a randomized controlled trial. Obstetrics and Gynecology 2008;112:1029‐36. - PubMed
Carbonell 2012 {published data only}
Carbonell 2013 {published data only}
Carbonell 2013a {published data only}
Chwalisz 2003 {published data only}
-
- Chwalisz K, Parker RL, Williamson S, Larsen L, McCrary K, Elger W. Treatment of uterine leiomyomas with the novel selective progesterone receptor modulator (SPRM). Journal of the Society for Gynecologic Investigation 2003;10:301A.
Donnez 2014 {published data only}
-
- Donnez J, Vazquez F, Tomaszewski J, Nouri K, Bouchard P, Fauser BCJM, et al. Long‐term treatment of uterine fibroids with ulipristal acetate. Fertility and Sterility 2014;101:1565‐73.e18. - PubMed
Eisinger 2003 {published data only}
-
- Eisinger SH, Meldrum S, Fiscella K, Roux HD, Guzick DS. Low‐dose mifepristone for uterine leiomyomata. Obstetrics & Gynecology 2003;101:243‐50. - PubMed
Eisinger 2005 {published data only}
-
- Eisinger SH, Bonfiglio T, Fiscella K, Meldrum S, Guzick DS. Twelve‐month safety and efficacy of low‐dose mifepristone for uterine myomas. Journal of Minimally Invasive Gynecology 2005;12:227‐33. - PubMed
Esteve 2012 {published data only}
-
- Esteve JL, Acosta R, Perez Y, Campos R, Hernandez AV, Texido CS. Treatment of uterine myoma with 5 or 10mg mifepristone daily during 6 months, post‐treatment evolution over 12 months: double‐blind randomised clinical trial. European Journal of Obstetrics, Gynecology, & Reproductive Biology 2012;161:202‐8. - PubMed
Kulshrestha 2013 {published data only}
References to studies awaiting assessment
NCT00044876 {published data only}
-
- NCT00044876. Treatment of uterine fibroids with CDB‐2914, an experimental selective progesterone receptor antagonist. https://clinicaltrials.gov/ct2/show/NCT00044876 (first received 5 September 2002).
NCT00152269 {published data only}
-
- NCT00152269. Treatment of uterine fibroids with asoprisnil (J867). https://clinicaltrials.gov/ct2/show/NCT00152269 (first received 7 September 2005).
NCT00683917 {published data only}
-
- NCT00683917. Pharmacokinetics, safety and efficacy study of Proellex in pre‐menopausal women with symptomatic uterine fibroids. https://clinicaltrials.gov/ct2/show/NCT00683917 (first received 22 May 2008).
NCT00702702 {published data only}
-
- NCT00702702. Safety and efficacy of Proellex in pre‐menopausal anemic women with symptomatic uterine fibroids. https://clinicaltrials.gov/ct2/show/NCT00702702 (first received 18 June 2008).
NCT00735553 {published data only}
-
- NCT00735553. Evaluating the safety and efficacy of Proellex (CDB‐4124) in premenopausal women with asymptomatic uterine fibroids. https://clinicaltrials.gov/ct2/show/NCT00735553 (first received 13 August 2008).
NCT00785356 {published data only}
-
- NCT00785356. Safety and efficacy of Proellex in pre‐menopausal anemic women with symptomatic uterine fibroids. https://clinicaltrialbase.com/study/NCT00785356 (first received 2 November 2008).
NCT00853567 {published data only}
-
- NCT00853567. Evaluating the safety and efficacy of Proellex in premenopausal women with symptomatic uterine fibroids. https://clinicaltrialbase.com/study/NCT00853567 (first received 26 February 2009).
NCT00882258 {published data only}
-
- NCT00882258. Study of Proellex in pre‐menopausal women with symptomatic uterine fibroids. https://clinicaltrials.gov/ct2/show/NCT00882258 (first received 14 April 2009).
NCT01069094 {published data only}
-
- NCT01069094. A Study of Progenta (CDB‐4124) in pre‐menopausal women with symptomatic leiomyomata. https://clinicaltrials.gov/ct2/show/NCT01069094 (first received 12 February 2010).
NCT01816815 {published data only}
-
- NCT01816815. Study in healthy tubal ligated women to evaluate pharmacodynamics, safety and pharmacokinetics of BAY1002670. https://clinicaltrials.gov/ct2/show/NCT01816815 (first received 20 March 2013).
References to ongoing studies
NCT02131662 {published data only}
-
- NCT02131662. Bay1002670, fibroids, safety and efficacy EU, US, Can, Jap (ASTEROID 1). https://clinicaltrials.gov/ct2/show/NCT02131662 (first received 5 May 2014).
NCT02147158 {published data only}
-
- NCT02147158. A study of the safety and efficacy of intermittent ulipristal treatment of abnormal uterine bleeding associated with leiomyomas. https://clinicaltrials.gov/ct2/show/NCT02147158 (first received 10 May 2014) 2016.
NCT02147197 {published data only}
-
- NCT02147197. A study of the efficacy and safety of a single ulipristal treatment course for the treatment of abnormal uterine bleeding associated with leiomyomas. https://clinicaltrials.gov/ct2/show/NCT02147197 (first received 21 May 2014).
NCT02288130 {published data only}
-
- NCT02288130. Ulipristal vs. GnRHa prior to laparoscopic myomectomy. https://clinicaltrials.gov/ct2/show/NCT02288130 (first received 6 November 2014).
NCT02323646 {published data only}
-
- NCT02323646. A phase 2 study to evaluate the safety and efficacy of Proellex (telapristone acetate) administered vaginally in the treatment of uterine fibroids. https://clinicaltrials.gov/ct2/show/NCT02323646 (first received 18 December 2014).
NCT02357563 {published data only}
-
- NCT02357563. Ulipristal acetate versus GnRH analogue and myometrial preservation. https://clinicaltrials.gov/ct2/show/NCT02357563 (first received 2 February 2015).
NCT02361879 {published data only}
-
- NCT02361879. Ulipristal acetate versus GnRH analogue treatment before hysteroscopic resection of uterine leiomyoma. https://clinicaltrials.gov/ct2/show/NCT02361879 (first received 2 February 2015).
NCT02361905 {published data only}
-
- NCT02361905. Ulipristal acetate for the preoperative management of hypoechoic cellular leiomyomas. https://clinicaltrials.gov/ct2/show/NCT02361905 (first received 2 February 2015).
NCT02425878 {published data only}
-
- NCT02425878. Ulipristal acetate 10 mg and assisted reproduction. https://clinicaltrials.gov/ct2/show/NCT02425878 (first received 21 April 2015).
NCT02465814 {published data only}
-
- NCT02465814. Assess safety and efficacy of vilaprisan in patients with uterine fibroids. https://clinicaltrials.gov/ct2/show/NCT02465814 (first received 22 May 2015).
Additional references
Baird 2003
-
- Baird DD, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. American Journal of Obstetrics and Gynecology 2003;188(1):100‐7. [PUBMED: 12548202] - PubMed
Bouchard 2011
-
- Bouchard P, Chabbert‐Buffet N, Fauser BC. Selective progesterone receptor modulators in reproductive medicine: pharmacology, clinical efficacy and safety. Fertility and Sterility 2011;96(5):1175‐89. [PUBMED: 21944187] - PubMed
Brooks 1996
-
- Brooks R. EuroQol: the current state of play. Health Policy 1996;37(1):53‐72. [PUBMED: 10158943] - PubMed
Bulun 2013
-
- Bulun SE. Uterine fibroids. New England Journal of Medicine 2013;369(14):1344‐55. [PUBMED: 24088094] - PubMed
Cardozo 2012
Chwalisz 2005
-
- Chwalisz K, Perez MC, Demanno D, Winkel C, Schubert G, Elger W. Selective progesterone receptor modulator development and use in the treatment of leiomyomata and endometriosis. Endocrine Reviews 2005;26(3):423‐38. [PUBMED: 15857972] - PubMed
Cohen 1988
-
- Cohen J. Statistical Power Analysis in the Behavioral Sciences. 2nd Edition. Hillsdale, NJ: Lawrence Erlbaum Associates, Inc., 1988.
Donnez 2015
-
- Donnez J, Hudecek R, Donnez O, Matule D, Arhendt HJ, Zatik J, et al. Efficacy and safety of repeated use of ulipristal acetate in uterine fibroids. Fertility and Sterility 2015;103(2):519‐27.e3. [PUBMED: 25542821] - PubMed
GRADEpro GDT 2014 [Computer program]
-
- GRADE Working Group, McMaster University. GRADEpro GDT. Version accessed before November 2016. Hamilton, ON: GRADE Working Group, McMaster University, 2014.
Gurusamy 2016
Higgins 2011
-
- Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Higham 1990
-
- Higham JM, O'Brien PMS, Shaw RW. Assessment of menstrual blood loss using a pictorial chart. BJOG 1990;97:734‐9. - PubMed
Ishikawa 2010
Kalampokas 2016
-
- Kalampokas T, Kamath M, Boutas I, Kalampokas E. Ulipristal acetate for uterine fibroids: a systematic review and meta‐analysis. Gynecological Endocrinology 2016;32(2):91‐6. [PUBMED: 26572056] - PubMed
Ke 2009
Khan 2014
-
- Khan K. The CROWN Initiative: journal editors invite researchers to develop core outcomes in women's health. BJOG 2014; Vol. 121, issue 10:1181‐2. [PUBMED: 24889142] - PubMed
Lethaby 2001
Merrill 2008
-
- Merrill RM. Hysterectomy surveillance in the United States, 1997 through 2005. Medical Science Monitor 2008;14(1):CR24‐31. [PUBMED: 18160941] - PubMed
Murji 2016
-
- Murji A, Crosier R, Chow T, Ye XY, Shirreff L. Role of ethnicity in treating uterine fibroids with ulipristal acetate. Fertility and Sterility 2016;106(5):1165‐9. [PUBMED: 27336213] - PubMed
Mutter 2008
-
- Mutter GL, Bergeron C, Deligdisch L, Ferenczy A, Glant M, Merino M, et al. The spectrum of endometrial pathology induced by progesterone receptor modulators. Modern Pathology 2008;21(5):591‐8. [PUBMED: 18246050] - PubMed
RevMan 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sabry 2012
Sangkomkamhang 2013
Spies 2002
-
- Spies JB, Coyne K, Guaou Guaou N, Boyle D, Skyrnarz‐Murphy K, Gonzalves SM. The UFS‐QOL, a new disease‐specific symptom and health‐related quality of life questionnaire for leiomyomata. Obstetrics and Gynecology 2002;99(2):290‐300. [PUBMED: 11814511] - PubMed
Stovall 2001
-
- Stovall DW. Clinical symptomatology of uterine leiomyomas. Clinical Obstetrics and Gynecology 2001;44(2):364‐71. [PUBMED: 11344999] - PubMed
Thrippleton 2015
Tristan 2012
Van Voorhis 2009
-
- Voorhis B. A 41‐year‐old woman with menorrhagia, anemia, and fibroids: review of treatment of uterine fibroids. JAMA 2009;301(1):82‐93. [PUBMED: 19050179] - PubMed
Wagenfeld 2016
-
- Wagenfeld A, Saunders PT, Whitaker L, Critchley HO. Selective progesterone receptor modulators (SPRMs): progesterone receptor action, mode of action on the endometrium and treatment options in gynecological therapies. Expert Opinion on Therapeutic Targets 2016;20(9):1‐10. [PUBMED: 27138351] - PMC - PubMed
Wallach 2004
-
- Wallach EE, Vlahos NF. Uterine myomas: an overview of development, clinical features, and management. Obstetrics and Gynecology 2004;104(2):393‐406. [PUBMED: 15292018] - PubMed
Ware 1992
-
- Ware JE Jr, Sherbourne CD. The MOS 36‐item short‐form health survey (SF‐36). I. Conceptual framework and item selection. Medical Care 1992;30(6):473‐83. [PUBMED: 1593914] - PubMed
Williams 2006
-
- Williams VS, Jones G, Mauskopf J, Spalding J, DuChane J. Uterine fibroids: a review of health‐related quality of life assessment. Journal of Womens Health 2006;15(7):818‐29. [PUBMED: 16999637] - PubMed
Wyatt 2001
-
- Wyatt KM, Dimmock PW, Walker TJ, O’Brien PMS. Determination of total menstrual blood loss. Fertility and Sterility 2001;76:125‐31. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

