Dual cyclin-dependent kinase 4/6 inhibition by PD-0332991 induces apoptosis and senescence in oesophageal squamous cell carcinoma cells
- PMID: 28444744
- PMCID: PMC5513862
- DOI: 10.1111/bph.13836
Dual cyclin-dependent kinase 4/6 inhibition by PD-0332991 induces apoptosis and senescence in oesophageal squamous cell carcinoma cells
Abstract
Background and purpose: Aberrant activation of the cyclin D1-cyclin-dependent kinase 4/6 (CDK4/6)-Rb signalling pathway is common in oesophageal squamous cell carcinoma (ESCC). PD-0332991, a highly specific inhibitor of CDK4/6, has potent antitumour activity against many types of cancer. The purpose of this study was to examine the in vitro and in vivo antineoplastic effect of PD-0332991 against the growth and metastasis of ESCC cells.
Experimental approach: Cell viability and any synergy between PD-0332991 and 5-fluorouracil or cisplatin were measured by MTS assay and CalcuSyn software respectively. Cell migration and invasion were detected by wound healing and transwell assays. Apoptosis was evaluated by flow cytometry after staining annexin V-FITC/PI. Cellular senescence was assessed by measuring SA-β-gal activity. Nude mouse xenograft models of ESCC were employed to determine the in vivo activity of PD-0332991 against tumour growth and lung metastasis.
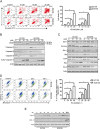
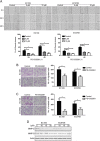
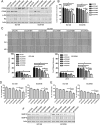
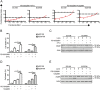
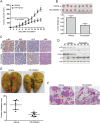
Key results: PD-0332991 inhibited cellular growth and induced mitochondrial-dependent apoptosis in ESCC cells. PD-0332991 also suppressed migration, invasion and the expression of MMP-2 in ESCC cells. Furthermore, PD-0332991 treatment caused cell senescence in a FOXM1-dependent manner. In addition, there was synergy between PD-0332991 and cisplatin or 5-fluorouracil. Importantly, the xenografted tumour experiments demonstrated that PD-0332991 potently inhibits ESCC cell growth and lung metastasis.
Conclusions and implications: PD-0332991 can elicit a strong antitumour activity against ESCC growth and metastasis and may be a promising candidate drug for the treatment of patients with ESCC. Our results warrant a clinical trial to further evaluate the efficacy of PD-0332991 in ESCC patients, even those with metastasis.
© 2017 The British Pharmacological Society.
Figures


References
-
- Baughn LB, Di Liberto M, Wu K, Toogood PL, Louie T, Gottschalk R et al. (2006). A novel orally active small molecule potently induces G1 arrest in primary myeloma cells and prevents tumor growth by specific inhibition of cyclin‐dependent kinase 4/6. Cancer Res 66: 7661–7667. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

