Earlier defibrotide initiation post-diagnosis of veno-occlusive disease/sinusoidal obstruction syndrome improves Day +100 survival following haematopoietic stem cell transplantation
- PMID: 28444784
- PMCID: PMC5635854
- DOI: 10.1111/bjh.14727
Earlier defibrotide initiation post-diagnosis of veno-occlusive disease/sinusoidal obstruction syndrome improves Day +100 survival following haematopoietic stem cell transplantation
Abstract
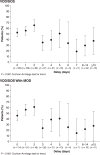
Hepatic veno-occlusive disease/sinusoidal obstruction syndrome (VOD/SOS) is a progressive, potentially fatal complication of conditioning for haematopoietic stem cell transplant (HSCT). The VOD/SOS pathophysiological cascade involves endothelial-cell activation and damage, and a prothrombotic-hypofibrinolytic state. Severe VOD/SOS (typically characterized by multi-organ dysfunction) may be associated with >80% mortality. Defibrotide is approved for treating severe hepatic VOD/SOS post-HSCT in the European Union, and for hepatic VOD/SOS with renal or pulmonary dysfunction post-HSCT in the United States. Previously, defibrotide (25 mg/kg/day in 4 divided doses for a recommended ≥21 days) was available through an expanded-access treatment protocol for patients with VOD/SOS. Data from this study were examined post-hoc to determine if the timing of defibrotide initiation post-VOD/SOS diagnosis affected Day +100 survival post-HSCT. Among 573 patients, defibrotide was started on the day of VOD/SOS diagnosis in approximately 30%, and within 7 days in >90%. The relationship between Day +100 survival and treatment initiation before/after specific days post-diagnosis showed superior survival when treatment was initiated closer to VOD/SOS diagnosis with a statistically significant trend over time for better outcomes with earlier treatment initiation (P < 0·001). These results suggest that initiation of defibrotide should not be delayed after diagnosis of VOD/SOS.
Keywords: defibrotide; sinusoidal obstruction syndrome; survival; treatment initiation; veno-occlusive disease.
© 2017 The Authors. British Journal of Haematology published by John Wiley & Sons Ltd.
Conflict of interest statement
Paul G. Richardson has served on an advisory committee and as a consultant to Jazz Pharmaceuticals and Gentium, and has received research funding from Gentium; Angela R. Smith has no relevant financial relationships to disclose; Brandon M. Triplett has no relevant financial relationships to disclose; Nancy A. Kernan has received research funding from Gentium; Nancy A. Kernan’s research was supported by National Cancer Institute of the National Institutes of Health under award number P30 CA008748; the content is solely the responsibility of the author and does not necessarily represent the official views of the National Institutes of Health; Stephan A. Grupp has served as a consultant to Jazz Pharmaceuticals; Joseph H. Antin has served on an advisory board for Jazz Pharmaceuticals and for Gentium; Leslie Lehmann has no relevant financial relationships to disclose; Maja Miloslavsky and Robin Hume are employees of Jazz Pharmaceuticals, who in the course of their employment have received stock options exercisable for, and other stock awards of, ordinary shares of Jazz Pharmaceuticals plc; Alison L. Hannah served as a consultant to Jazz Pharmaceuticals; Bijan Nejadnik is a former employee of Jazz Pharmaceuticals, who in the course of his employment had received stock options exercisable for, and other stock awards of, ordinary shares of Jazz Pharmaceuticals plc; Robert J. Soiffer has served on an advisory board for Gentium and Jazz Pharmaceuticals.
Figures
Similar articles
-
Defibrotide for Patients with Hepatic Veno-Occlusive Disease/Sinusoidal Obstruction Syndrome: Interim Results from a Treatment IND Study.Biol Blood Marrow Transplant. 2017 Jun;23(6):997-1004. doi: 10.1016/j.bbmt.2017.03.008. Epub 2017 Mar 8. Biol Blood Marrow Transplant. 2017. PMID: 28285079
-
Final results from a defibrotide treatment-IND study for patients with hepatic veno-occlusive disease/sinusoidal obstruction syndrome.Br J Haematol. 2018 Jun;181(6):816-827. doi: 10.1111/bjh.15267. Epub 2018 May 16. Br J Haematol. 2018. PMID: 29767845 Free PMC article. Clinical Trial.
-
Defibrotide for the treatment of hepatic veno-occlusive disease/sinusoidal obstruction syndrome following nontransplant-associated chemotherapy: Final results from a post hoc analysis of data from an expanded-access program.Pediatr Blood Cancer. 2018 Oct;65(10):e27269. doi: 10.1002/pbc.27269. Epub 2018 Jun 6. Pediatr Blood Cancer. 2018. PMID: 29873895 Free PMC article. Clinical Trial.
-
Defibrotide sodium for the treatment of hepatic veno-occlusive disease/sinusoidal obstruction syndrome.Expert Rev Clin Pharmacol. 2018 Feb;11(2):113-124. doi: 10.1080/17512433.2018.1421943. Epub 2018 Jan 5. Expert Rev Clin Pharmacol. 2018. PMID: 29301447 Review.
-
The use of defibrotide in blood and marrow transplantation.Blood Adv. 2018 Jun 26;2(12):1495-1509. doi: 10.1182/bloodadvances.2017008375. Blood Adv. 2018. PMID: 29945939 Free PMC article. Review.
Cited by
-
Ultrasound elastography techniques for diagnosis and follow-up of hepatic veno-occlusive disease.Bone Marrow Transplant. 2019 Jul;54(7):1145-1147. doi: 10.1038/s41409-019-0432-5. Epub 2019 Jan 24. Bone Marrow Transplant. 2019. PMID: 30679827 Free PMC article. No abstract available.
-
Influence of creatinine levels on survival in patients with veno-occlusive disease treated with defibrotide.Korean J Intern Med. 2022 Jan;37(1):179-189. doi: 10.3904/kjim.2021.178. Epub 2021 Dec 14. Korean J Intern Med. 2022. PMID: 34902236 Free PMC article.
-
Prevention and management of acute toxicities from conditioning regimens during hematopoietic stem cell transplantation.Clin Hematol Int. 2024 Apr 3;6(2):1-10. doi: 10.46989/001c.94952. eCollection 2024. Clin Hematol Int. 2024. PMID: 38817311 Free PMC article. Review.
-
Classical and Late-Onset SOS/VOD After Allogeneic HSCT: A Japanese Transplant Registry Analysis.Am J Hematol. 2025 Aug;100(8):1283-1294. doi: 10.1002/ajh.27715. Epub 2025 May 19. Am J Hematol. 2025. PMID: 40387331 Free PMC article.
-
Prophylactic, preemptive, and curative treatment for sinusoidal obstruction syndrome/veno-occlusive disease in adult patients: a position statement from an international expert group.Bone Marrow Transplant. 2020 Mar;55(3):485-495. doi: 10.1038/s41409-019-0705-z. Epub 2019 Oct 1. Bone Marrow Transplant. 2020. PMID: 31576023 Free PMC article.
References
-
- Bearman SI. The syndrome of hepatic veno-occlusive disease after marrow transplantation. Blood. 1995;85:3005–3020. - PubMed
-
- Benimetskaya L, Wu S, Voskresenskiy AM, Echart C, Zhou JF, Shin J, Iacobelli M, Richardson P, Ayyanar K, Stein CA. Angiogenesis alteration by defibrotide: implications for its mechanism of action in severe hepatic veno-occlusive disease. Blood. 2008;112:4343–4352. - PubMed
-
- Carreras E, Diaz-Ricart M. The role of the endothelium in the short-term complications of hematopoietic SCT. Bone Marrow Transplantation. 2011;46:1495–1502. - PubMed
-
- Carreras E, Díaz-Beyá M, Rosiñol L, Martínez C, Fernández-Avilés F, Rovira M. The incidence of veno-occlusive disease following allogeneic hematopoietic stem cell transplantation has diminished and the outcome improved over the last decade. Biology of Blood and Marrow Transplantation. 2011;17:1713–1720. - PubMed
-
- Coppell JA, Richardson PG, Soiffer R, Martin PL, Kernan NA, Chen A, Guinan E, Vogelsang G, Krishnan A, Giralt S, Revta C, Carreau NA, Iacobelli M, Carreras E, Ruutu T, Barbui T, Antin JH, Niederwieser D. Hepatic veno-occlusive disease following stem cell transplantation: incidence, clinical course, and outcome. Biology of Blood and Marrow Transplantation. 2010;16:157–168. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

