Synergistic Visible-Light Photoredox/Nickel-Catalyzed Synthesis of Aliphatic Ketones via N-C Cleavage of Imides
- PMID: 28445061
- PMCID: PMC5423445
- DOI: 10.1021/acs.orglett.7b00989
Synergistic Visible-Light Photoredox/Nickel-Catalyzed Synthesis of Aliphatic Ketones via N-C Cleavage of Imides
Abstract
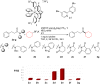
An electrophilic, imide-based, visible-light-promoted photoredox/Ni-catalyzed cross-coupling reaction for the synthesis of aliphatic ketones has been developed. This protocol proceeds through N-C(O) bond activation, made possible through the lower activation energy for metal insertion into this bond due to delocalization of the lone pair of electrons on the nitrogen by electron-withdrawing groups. The operationally simple and mild cross-coupling reaction is performed at ambient temperature and exhibits tolerance for a variety of functional groups.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



Similar articles
-
Synergistic Photoredox/Nickel Coupling of Acyl Chlorides with Secondary Alkyltrifluoroborates: Dialkyl Ketone Synthesis.J Org Chem. 2017 Feb 3;82(3):1856-1863. doi: 10.1021/acs.joc.6b02897. Epub 2017 Jan 17. J Org Chem. 2017. PMID: 28093913 Free PMC article.
-
Silyl Radical Mediated Cross-Electrophile Coupling of N-Acyl-imides with Alkyl Bromides under Photoredox/Nickel Dual Catalysis.Org Lett. 2020 Mar 20;22(6):2240-2245. doi: 10.1021/acs.orglett.0c00442. Epub 2020 Mar 9. Org Lett. 2020. PMID: 32148046
-
Single-Electron Transmetalation: Photoredox/Nickel Dual Catalytic Cross-Coupling of Secondary Alkyl β-Trifluoroboratoketones and -esters with Aryl Bromides.Org Lett. 2016 Jun 17;18(12):2994-7. doi: 10.1021/acs.orglett.6b01357. Epub 2016 Jun 6. Org Lett. 2016. PMID: 27265019
-
Nickel-Catalyzed C-Heteroatom Cross-Coupling Reactions under Mild Conditions via Facilitated Reductive Elimination.Angew Chem Int Ed Engl. 2021 Aug 9;60(33):17810-17831. doi: 10.1002/anie.202013852. Epub 2021 Feb 25. Angew Chem Int Ed Engl. 2021. PMID: 33252192 Review.
-
At the Speed of Light: The Systematic Implementation of Photoredox Cross-Coupling Reactions for Medicinal Chemistry Research.J Org Chem. 2024 Nov 15;89(22):16070-16092. doi: 10.1021/acs.joc.3c02351. Epub 2024 Mar 5. J Org Chem. 2024. PMID: 38442262 Review.
Cited by
-
Nickel-Mediated Photoreductive Cross Coupling of Carboxylic Acid Derivatives for Ketone Synthesis.Chemistry. 2021 Dec 23;27(72):18168-18174. doi: 10.1002/chem.202103486. Epub 2021 Nov 23. Chemistry. 2021. PMID: 34709698 Free PMC article.
-
Metallaphotoredox: The Merger of Photoredox and Transition Metal Catalysis.Chem Rev. 2022 Jan 26;122(2):1485-1542. doi: 10.1021/acs.chemrev.1c00383. Epub 2021 Nov 18. Chem Rev. 2022. PMID: 34793128 Free PMC article. Review.
-
Stereochemistry of Transition Metal Complexes Controlled by the Metallo-Anomeric Effect.J Am Chem Soc. 2020 Sep 2;142(35):15127-15136. doi: 10.1021/jacs.0c06882. Epub 2020 Aug 19. J Am Chem Soc. 2020. PMID: 32786781 Free PMC article.
-
Direct Conversion of Carboxylic Acids to Alkyl Ketones.Org Lett. 2017 Jul 7;19(13):3612-3615. doi: 10.1021/acs.orglett.7b01588. Epub 2017 Jun 12. Org Lett. 2017. PMID: 28604003 Free PMC article.
-
Ni-Catalyzed Suzuki-Miyaura Cross-Coupling of Aliphatic Amides on the Benchtop.Org Lett. 2020 Jan 3;22(1):1-5. doi: 10.1021/acs.orglett.9b03434. Epub 2019 Oct 17. Org Lett. 2020. PMID: 31621338 Free PMC article.
References
-
- Blangetti M.; Rosso H.; Prandi C.; Deagostino A.; Venturello P. Molecules 2013, 18, 1188.10.3390/molecules18011188. - DOI - PMC - PubMed
- Urawa Y.; Ogura K. Tetrahedron Lett. 2003, 44, 271.10.1016/S0040-4039(02)02501-7. - DOI
- Haddach M.; McCarthy J. R. Tetrahedron Lett. 1999, 40, 3109.10.1016/S0040-4039(99)00476-1. - DOI
- Bumagin N. A.; Korolev D. N. Tetrahedron Lett. 1999, 40, 3057.10.1016/S0040-4039(99)00364-0. - DOI
-
- Gooßen L. J.; Ghosh K. Angew. Chem., Int. Ed. 2001, 40, 3458.10.1002/1521-3773(20010917)40:18<3458::AID-ANIE3458>3.0.CO;2-0. - DOI - PubMed
- Kakino R.; Yasumi S.; Shimizu I.; Yamamoto A. Bull. Chem. Soc. Jpn. 2002, 75, 137.10.1246/bcsj.75.137. - DOI
- Kakino R.; Shimizu I.; Yamamoto A. Bull. Chem. Soc. Jpn. 2001, 74, 371.10.1246/bcsj.74.371. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

