Therapeutic benefit of Salmonella attributed to LPS and TNF-α is exhaustible and dictated by tumor susceptibility
- PMID: 28445131
- PMCID: PMC5482671
- DOI: 10.18632/oncotarget.16906
Therapeutic benefit of Salmonella attributed to LPS and TNF-α is exhaustible and dictated by tumor susceptibility
Abstract
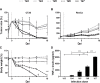
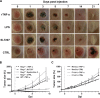
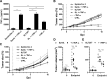
The potential of bacteria-mediated tumor therapy (BMTT) is highlighted by more than a century of investigation. Attenuated Salmonella has prevailed as promising therapeutic agents. For BMTT - categorized as an immune therapy - the exact contribution of particular immune reactions to the therapeutic effect remains ambiguous. In addition, one could argue for or against the requirement of bacterial viability and tumor targeting. Herein we evaluate the isolated therapeutic efficacy of purified LPS and TNF-α, which together account for a dominant immunogenic pathway of gram negative bacteria like Salmonella. We show that therapeutic efficacy against CT26 tumors does not require bacterial viability. Analogous to viable Salmonella SL7207, tumor regression by a specific CD8+ T cell response can be induced by purified LPS or recombinant TNF-α (rTNF-α). Conversely, therapeutic effects against RenCa tumors were abrogated upon bacterial avitalization and limited using isolated adjuvants. This argues for an alternative mechanistic explanation for SL7207 against RenCa that depends on viability and persistence. Unable to boost bacterial therapies by co-injection of rTNF-α suggested therapeutic effects along this axis are exhausted by the intrinsic adjuvanticity of bacteria alone. However, the importance of TNF-α for BMTT was highlighted by its support of tumor invasion and colonization in concert with lower infective doses of Salmonella. In consideration, bacterial therapeutic effectiveness along the axis of LPS and TNF-α appears limited, and does not offer the necessary plasticity for different tumors. This emphasizes a need for recombinant strengthening and vehicular exploitation to accommodate potency, plasticity and distinctiveness in BMTT.
Keywords: LPS; TNF alpha; bacteria mediated tumor therapy; cancer immune therapy; salmonella.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

