Monitoring of KRAS-mutated ctDNA to discriminate pseudo-progression from true progression during anti-PD-1 treatment of lung adenocarcinoma
- PMID: 28445137
- PMCID: PMC5514971
- DOI: 10.18632/oncotarget.16935
Monitoring of KRAS-mutated ctDNA to discriminate pseudo-progression from true progression during anti-PD-1 treatment of lung adenocarcinoma
Abstract
Objectives: Pseudo-progression is a rare but worrying situation for both clinicians and patients during immunotherapy. Dedicated ir-RECIST criteria have been established to improve this situation. However, this can be sometimes considered inadequate and patients experiencing true progression may then receive inefficient treatments. Additional reliable tools to discriminate pseudo from true progression are thus needed. So far, no biomarker has been identified to distinguish pseudo from true progression. We hypothesize that biomarkers associated with the molecular characteristics of the tumor may be of interest. To avoid a tumor re-biopsy, circulating markers appear to be a less invasive and reproducible procedure. As ctDNA kinetics correlate with the response to treatment in KRAS-mutated adenocarcinoma, we anticipated that this analysis could be of interest.
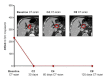
Materials and methods: We monitored the level of KRAS-mutated ctDNA by digital droplet PCR in serial plasma samples from two patients who had experienced pseudo-progression and compared the variations with those from of a patient that had true progression.
Results: ctDNA showed rapid and dramatic decreases in pseudo-progressive patients, whereas it was strongly increased in the progressive patient.
Conclusions: ddPCR of ctDNA may thus be an additional tool to discriminate pseudo-progression from true progression for tumors that harbor an oncogenic addiction.
Keywords: KRAS mutation; anti-PD-1; circulating tumor DNA; immunotherapy; non-small-cell lung cancer.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures


References
-
- Guibert N, Mazières J. Nivolumab for treating non-small cell lung cancer. Expert Opin Biol Ther. 2015;15:1789–97. - PubMed
-
- Wolchok JD, Hoos A, O’Day S, Weber JS, Hamid O, Lebbé C, Maio M, Binder M, Bohnsack O, Nichol G, Humphrey R, Hodi FS. Guidelines for the evaluation of immune therapy activity in solid tumors: Immune-related response criteria. Clin Cancer Res. 2009;15:7412–7420. - PubMed
-
- Hodi FS, Sznol M, Kluger HM, McDermott DF, Carvajal RD, Lawrence DP, Topalian SL, Atkins MB, Powderly JD, Sharfman WH, Puzanov I, Smith DC, Leming PD, et al. Long term survival of ipilimumab-naive patients with advanced melanoma treated with nivolumab (anti-PD-1, BMS-936558, ONO-4538) in a phase I trial. J Clin Oncol. 2014;15 abstr 9002.
-
- Hodi SF, Ribas A, Daud A, Hamid O, Robert C, Kefford R, Hwu WJ, Gangadhar TC, Joshua AM, Hersey P, Weber JS, Dronca RS, Perrone AM, et al. Evaluation of immune-related response criteria (irRC) in patients (pts) with advanced melanoma (MEL) treated with the anti-PD-1 monoclonal antibody MK-3475. J Clin Oncol. 2014;15 abstr 3006. - PMC - PubMed
-
- Guibert N, Pradines A, Farella M, Casanova A, Gouin S, Keller L, Favre G, Mazieres J. Monitoring KRAS mutations in circulating DNA and tumor cells using digital droplet PCR during treatment of KRAS-mutated lung adenocarcinoma. Lung Cancer. 2016;100:1–4. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

