Role of Delta-like 4 in Jagged1-induced tumour angiogenesis and tumour growth
- PMID: 28445154
- PMCID: PMC5522274
- DOI: 10.18632/oncotarget.16969
Role of Delta-like 4 in Jagged1-induced tumour angiogenesis and tumour growth
Abstract
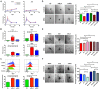
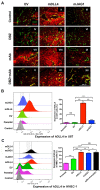
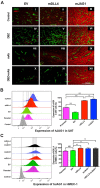
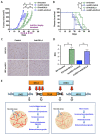
Delta-like 4 (DLL4) and Jagged1 (JAG1) are two key Notch ligands implicated in tumour angiogenesis. They were shown to have opposite effects on mouse retinal and adult regenerative angiogenesis. In tumours, both ligands are upregulated but their relative effects and interactions in tumour biology, particularly in tumour response to therapeutic intervention are unclear. Here we demonstrate that DLL4 and JAG1 displayed equal potency in stimulating Notch target genes in HMEC-1 endothelial cells but had opposing effects on sprouting angiogenesis in vitro. Mouse DLL4 or JAG1 expressed in glioblastoma cells decreased tumour cell proliferation in vitro but promoted tumour growth in vivo. mDLL4-expressing tumours showed fewer but larger vessels whereas mJAG1-tumours produced more vessels. In both tumour types pericyte coverage was decreased but the vessels were more perfused. Both ligands increased tumour resistance towards anti-VEGF therapy but the resistance was higher in mDLL4-tumours versus mJAG1-tumours. However, their sensitivity to the therapy was restored by blocking Notch signalling with dibenzazepine. Importantly, anti-DLL4 antibody blocked the effect of JAG1 on tumour growth and increased vessel branching in vivo. The mechanism behind the differential responsiveness was due to a positive feedback loop for DLL4-Notch signalling, rendering DLL4 more dominant in activating Notch signalling in the tumour microenvironment. We concluded that DLL4 and JAG1 promote tumour growth by modulating tumour angiogenesis via different mechanisms. JAG1 is not antagonistic but utilises DLL4 in tumour angiogenesis. The results suggest that anti-JAG1 therapy should be explored in conjunction with anti-DLL4 treatment in developing anti-Notch therapies in clinics.
Keywords: DLL4; JAG1; Notch signalling; angiogenesis; bevacizumab.
Conflict of interest statement
The authors declare no potential conflicts of interests.
Figures



References
-
- Li JL, Harris AL. Crosstalk of VEGF and Notch pathways in tumour angiogenesis: therapeutic implications. Front Biosci. 2009;14:3094–3110. - PubMed
-
- Gale NW, Dominguez MG, Noguera I, Pan L, Hughes V, Valenzuela DM, Murphy AJ, Adams NC, Lin HC, Holash J, Thurston G, Yancopoulos GD. Haploinsufficiency of delta-like 4 ligand results in embryonic lethality due to major defects in arterial and vascular development. Proc Natl Acad Sci U S A. 2004;101:15949–15954. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

