miR-103 Promotes Proliferation and Metastasis by Targeting KLF4 in Gastric Cancer
- PMID: 28445396
- PMCID: PMC5454823
- DOI: 10.3390/ijms18050910
miR-103 Promotes Proliferation and Metastasis by Targeting KLF4 in Gastric Cancer
Abstract
MicroRNAs (miRNAs) play important roles in the cancer development and progression; overexpression of miR-103 has been identified in various tumors. However, its biological function and regulatory mechanism involved in modulation of human gastric cancer (GC) remain largely unknown. This study aimed to confirm clinical significance of miR-103 and investigate its biological role and underlying mechanism in GC. Real-time quantitative PCR (qRT-PCR) revealed miR-103 was highly expressed in GC tissues and cell lines. miR-103 expression was correlated closely with tumor size, Lauren's classification, and lymph node metastasis. Importantly, Kaplan-Meier analysis revealed that high expression of miR-103 was significantly associated with poor overall survival and disease-free survival of GC patients. Downregulation of miR-103 by transfecting with miR-103 inhibitor significantly suppressed cell proliferation, induced apoptosis, inhibited migration and invasion in vitro and in vivo. Furthermore, miRNA target databases and luciferase reporter assay confirmed that Krüppel-like Factor-4 (KLF4) was a direct target of miR-103 in GC, and there was a significant inverse correlation between miR-103 and KLF4 expression in GC tissues. Moreover, KLF4 downregulation could rescue miR-103's oncogenic effect on GC cell proliferation, apoptosis, migration, and invasion. Therefore, these results suggested that miR-103 overexpression could contribute to tumor progression by suppressing KLF4, and it might serve as a promising candidate for the prognosis of GC patients.
Keywords: KLF4; gastric cancer; metastasis; microRNA-103; proliferation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


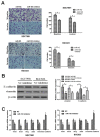
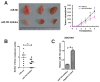
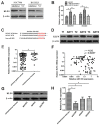
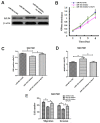
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

