Targeting the Epidermal Growth Factor Receptor in Addition to Chemotherapy in Patients with Advanced Pancreatic Cancer: A Systematic Review and Meta-Analysis
- PMID: 28445400
- PMCID: PMC5454822
- DOI: 10.3390/ijms18050909
Targeting the Epidermal Growth Factor Receptor in Addition to Chemotherapy in Patients with Advanced Pancreatic Cancer: A Systematic Review and Meta-Analysis
Abstract
Overexpression of epidermal growth factor receptors (EGFR) occurs in >90% of pancreatic ductal adenocarcinomas (PDACs) and is associated with a poorer prognosis. A systematic review of electronic databases identified studies exploring the addition of EGFR-targeted treatment to chemotherapy in patients with locally advanced (LA)/metastatic PDAC. Efficacy, safety and tolerability of EGFR-targeted therapy were explored using meta-analysis of randomised controlled trials (RCTs). Meta-regression was utilised to explore factors associated with improved prognosis (all studies) and benefit from EGFR-targeted therapy (RCTs). Twenty-eight studies (7 RCTs and 21 cohort studies) comprising 3718 patients were included. The addition of EGFR-targeted treatment to chemotherapy did not improve progression-free (pooled hazard ratio (HR): 0.90, p = 0.15) or overall survival (HR: 0.94, p = 0.18). EGFR-targeted therapy was associated with increased treatment-related deaths (pooled odds ratio (OR): 5.18, p = 0.007), and grade (G)3/4 rash (OR: 4.82, p = 0.03). There was a borderline significant increase in G3/4 diarrhoea (OR: 1.75, p = 0.06), but no effect on treatment discontinuation without progression (OR: 0.87, p = 0.25). Neither G3/4 rash nor diarrhoea were associated with increased survival benefit from EGFR-targeted therapy. The effect of EGFR-targeted therapy on overall survival (OS) appeared greater in studies with a greater proportion of LA rather than metastatic patients (R = -0.69, p < 0.001). Further studies in unselected patients with advanced PDAC are not warranted. The benefit from EGFR inhibitors may be limited to patient subgroups not yet clearly defined.
Keywords: KRAS; advanced pancreatic cancer; chemotherapy; epidermal growth factor receptors (EGFR); rash.
Conflict of interest statement
The authors report no conflict of interest in this work.
Figures



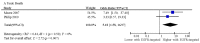
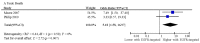
Similar articles
-
Epidermal growth factor receptor (EGFR) inhibitors for metastatic colorectal cancer.Cochrane Database Syst Rev. 2017 Jun 27;6(6):CD007047. doi: 10.1002/14651858.CD007047.pub2. Cochrane Database Syst Rev. 2017. PMID: 28654140 Free PMC article.
-
Gefitinib for advanced non-small cell lung cancer.Cochrane Database Syst Rev. 2018 Jan 16;1(1):CD006847. doi: 10.1002/14651858.CD006847.pub2. Cochrane Database Syst Rev. 2018. PMID: 29336009 Free PMC article.
-
The effectiveness and cost-effectiveness of carmustine implants and temozolomide for the treatment of newly diagnosed high-grade glioma: a systematic review and economic evaluation.Health Technol Assess. 2007 Nov;11(45):iii-iv, ix-221. doi: 10.3310/hta11450. Health Technol Assess. 2007. PMID: 17999840
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of topotecan for ovarian cancer.Health Technol Assess. 2001;5(28):1-110. doi: 10.3310/hta5280. Health Technol Assess. 2001. PMID: 11701100
-
Treatment options for progression or recurrence of glioblastoma: a network meta-analysis.Cochrane Database Syst Rev. 2021 May 4;5(1):CD013579. doi: 10.1002/14651858.CD013579.pub2. Cochrane Database Syst Rev. 2021. PMID: 34559423 Free PMC article.
Cited by
-
Preclinical Safety Evaluation of Intraperitoneally Administered Cu-Conjugated Anti-EGFR Antibody NCAB001 for the Early Diagnosis of Pancreatic Cancer Using PET.Pharmaceutics. 2022 Sep 12;14(9):1928. doi: 10.3390/pharmaceutics14091928. Pharmaceutics. 2022. PMID: 36145676 Free PMC article.
-
Process to Remove the Size Variants Contained in the Antibody-Chelator Complex PCTA-NCAB001 for Radiolabeling with Copper-64.Pharmaceuticals (Basel). 2023 Sep 22;16(10):1341. doi: 10.3390/ph16101341. Pharmaceuticals (Basel). 2023. PMID: 37895812 Free PMC article.
-
LncRNA-BLACAT1 Facilitates Proliferation, Migration and Aerobic Glycolysis of Pancreatic Cancer Cells by Repressing CDKN1C via EZH2-Induced H3K27me3.Front Oncol. 2020 Sep 23;10:539805. doi: 10.3389/fonc.2020.539805. eCollection 2020. Front Oncol. 2020. PMID: 33072570 Free PMC article.
-
EGFR-Pak Signaling Selectively Regulates Glutamine Deprivation-Induced Macropinocytosis.Dev Cell. 2019 Aug 5;50(3):381-392.e5. doi: 10.1016/j.devcel.2019.05.043. Epub 2019 Jun 27. Dev Cell. 2019. PMID: 31257175 Free PMC article.
-
Recent Advances in Small Molecular PET Tracers for Pancreatic Cancer Diagnosis: Preclinical Stage.Mini Rev Med Chem. 2025;25(10):745-759. doi: 10.2174/0113895575375382250408143606. Mini Rev Med Chem. 2025. PMID: 40247799 Review.
References
-
- De Santis C., Lin E. Cancer treatment and survivorship statistics. CA Cancer J. Clin. 2014;64:252–271. - PubMed
-
- World Cancer Research Fund International Pancreatic Cancer Statistics. [(accessed on 5 November 2016)]; Available online: www.wcrf.org/int/cancer-facts-figures/data-specific-cancers/pancreatic-c....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

