Discovery of Farnesoid X Receptor Antagonists Based on a Library of Oleanolic Acid 3-O-Esters through Diverse Substituent Design and Molecular Docking Methods
- PMID: 28445411
- PMCID: PMC6154651
- DOI: 10.3390/molecules22050690
Discovery of Farnesoid X Receptor Antagonists Based on a Library of Oleanolic Acid 3-O-Esters through Diverse Substituent Design and Molecular Docking Methods
Abstract
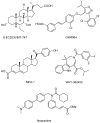
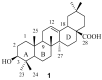
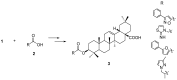
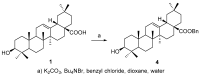
The pentacyclic triterpene oleanolic acid (OA, 1) with known farnesoid X receptor (FXR) modulatory activity was modified at its C-3 position to find new FXR-interacting agents. A diverse substitution library of OA derivatives was constructed in silico through a 2D fingerprint similarity cluster strategy. With further docking analysis, four top-scored OA 3-O-ester derivatives were selected for synthesis. The bioassay results indicated that all four compounds 3 inhibited chenodeoxycholic acid (CDCA)-induced FXR transactivation in a concentration-dependent mode. Among them 3b and 3d are more active than the parent compound OA. A molecular simulation study was performed to attempt to explain the structure-activity relationship (SAR) and the antagonistic action. To the best of our knowledge, this is the first report on semi-synthetic pentacyclic triterpenoids with FXR-modulatory activities.
Keywords: FXR antagonist; compound diversity; library design; molecular modeling; oleanolic acid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Harnessing Oleanolic Acid and Its Derivatives as Modulators of Metabolic Nuclear Receptors.Biomolecules. 2023 Sep 28;13(10):1465. doi: 10.3390/biom13101465. Biomolecules. 2023. PMID: 37892147 Free PMC article. Review.
-
Identification of 15d-PGJ2 as an antagonist of farnesoid X receptor: molecular modeling with biological evaluation.Steroids. 2013 Sep;78(9):813-22. doi: 10.1016/j.steroids.2013.04.018. Epub 2013 May 23. Steroids. 2013. PMID: 23707573
-
Discovery of new non-steroidal farnesoid X receptor modulators through 3D shape similarity search and structure-based virtual screening.Chem Biol Drug Des. 2015 Apr;85(4):481-7. doi: 10.1111/cbdd.12432. Epub 2014 Oct 10. Chem Biol Drug Des. 2015. PMID: 25228339
-
Oleanolic acid is a selective farnesoid X receptor modulator.Phytother Res. 2010 Mar;24(3):369-73. doi: 10.1002/ptr.2948. Phytother Res. 2010. PMID: 19653193
-
Oleanolic Acid and Its Derivatives: Biological Activities and Therapeutic Potential in Chronic Diseases.Molecules. 2017 Nov 13;22(11):1915. doi: 10.3390/molecules22111915. Molecules. 2017. PMID: 29137205 Free PMC article. Review.
Cited by
-
The Role of Five-Membered Aromatic Rings Containing N and O in Modulating Bile Acid Receptors: An Overview.ChemMedChem. 2025 Aug 16;20(16):e202500405. doi: 10.1002/cmdc.202500405. Epub 2025 Jul 11. ChemMedChem. 2025. PMID: 40536013 Free PMC article. Review.
-
Harnessing Oleanolic Acid and Its Derivatives as Modulators of Metabolic Nuclear Receptors.Biomolecules. 2023 Sep 28;13(10):1465. doi: 10.3390/biom13101465. Biomolecules. 2023. PMID: 37892147 Free PMC article. Review.
-
Prophylactic and therapeutic roles of oleanolic acid and its derivatives in several diseases.World J Clin Cases. 2020 May 26;8(10):1767-1792. doi: 10.12998/wjcc.v8.i10.1767. World J Clin Cases. 2020. PMID: 32518769 Free PMC article. Review.
-
The role of botanical triterpenoids and steroids in bile acid metabolism, transport, and signaling: Pharmacological and toxicological implications.Acta Pharm Sin B. 2024 Aug;14(8):3385-3415. doi: 10.1016/j.apsb.2024.04.027. Epub 2024 May 3. Acta Pharm Sin B. 2024. PMID: 39220868 Free PMC article. Review.
-
Conformational Characterization of the Co-Activator Binding Site Revealed the Mechanism to Achieve the Bioactive State of FXR.Front Mol Biosci. 2021 Aug 31;8:658312. doi: 10.3389/fmolb.2021.658312. eCollection 2021. Front Mol Biosci. 2021. PMID: 34532338 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

