Discovery of Farnesoid X Receptor Antagonists Based on a Library of Oleanolic Acid 3-O-Esters through Diverse Substituent Design and Molecular Docking Methods
- PMID: 28445411
- PMCID: PMC6154651
- DOI: 10.3390/molecules22050690
Discovery of Farnesoid X Receptor Antagonists Based on a Library of Oleanolic Acid 3-O-Esters through Diverse Substituent Design and Molecular Docking Methods
Abstract
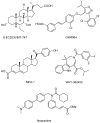
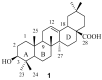
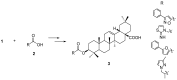
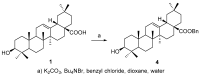
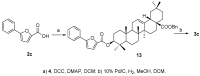
The pentacyclic triterpene oleanolic acid (OA, 1) with known farnesoid X receptor (FXR) modulatory activity was modified at its C-3 position to find new FXR-interacting agents. A diverse substitution library of OA derivatives was constructed in silico through a 2D fingerprint similarity cluster strategy. With further docking analysis, four top-scored OA 3-O-ester derivatives were selected for synthesis. The bioassay results indicated that all four compounds 3 inhibited chenodeoxycholic acid (CDCA)-induced FXR transactivation in a concentration-dependent mode. Among them 3b and 3d are more active than the parent compound OA. A molecular simulation study was performed to attempt to explain the structure-activity relationship (SAR) and the antagonistic action. To the best of our knowledge, this is the first report on semi-synthetic pentacyclic triterpenoids with FXR-modulatory activities.
Keywords: FXR antagonist; compound diversity; library design; molecular modeling; oleanolic acid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

